Professor Hywel C. Williams, Director of the NIHR Health Technology Assessment (HTA) Programme, sheds light on the HTA Programme that is pushing and pulling research that is needed by the UK National Health Service
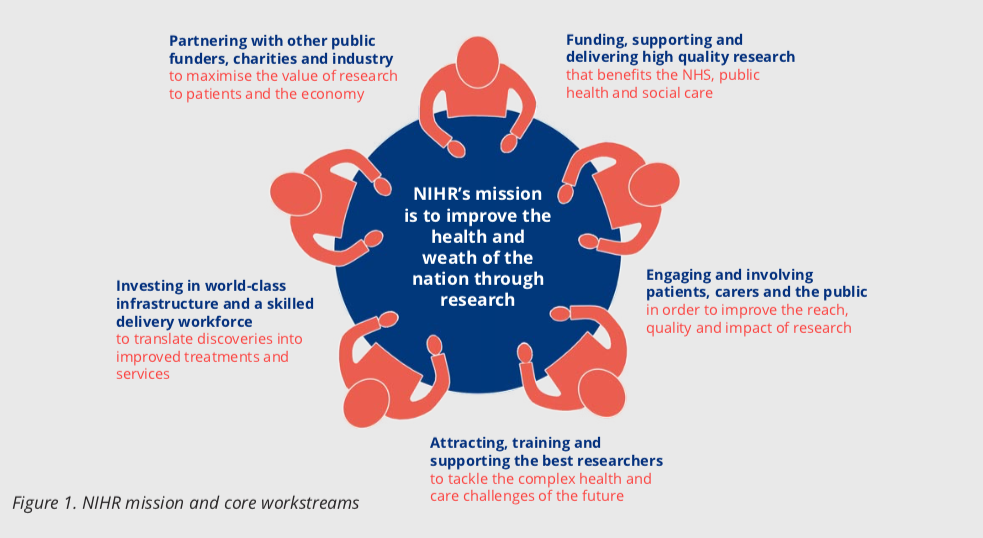
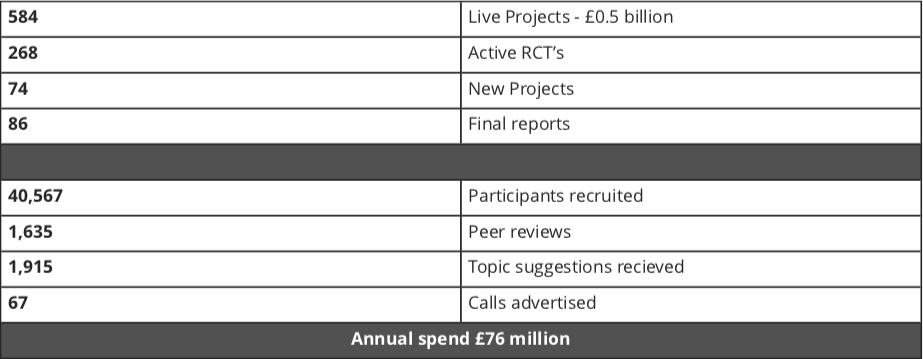
The NIHR Health Technology Assessment (HTA) Programme sits within the National Institute for Health Research (NIHR) (Figure 1) the UK’s largest funder of health and social care research, and is funded by the UK Government. The HTA Programme is the NIHR’s largest research programme. In 2018/19, the HTA Programme included 584 live projects worth £0.5 billion composed of 268 active clinical trials, 74 new projects, 86 final reports, 40,567 participants recruited, 1,635 peer reviewers, 1,915 topic suggestions received and 67 calls advertised with an annual spend £76 million.
‘Health technology assessment’ means research about the clinical and cost-effectiveness of healthcare treatments for those who plan, provide or receive care from NHS and social care services. The portfolio includes primary research (clinical trials) and secondary research (evidence syntheses) on any technology used to promote health, prevent and treat disease and improve rehabilitation or long-term care.
The ’technology’ in this sense includes drugs, talking therapies, procedures, diagnostic tests, devices and complex interventions, and spans the whole of medicine, surgery, mental health and social care. HTA sits towards the end of the technology development and assessment pathway – in other words, the technologies being assessed already have a good efficacy signal. What HTA does is to see how well they work in terms of effectiveness and cost-effectiveness when rolled out in a pragmatic national evaluation. An HTA project assesses whether a technology works, for whom, at what cost and how it compares with existing treatments.
How NIHR HTA funds research: Pushing and pulling research that is needed
A unique aspect of the HTA Programme since its inception in 1993 is its role in commissioning research that is needed by the NHS. These include interventions that would never be cost-effective for industry to evaluate, but which nevertheless provide large benefits to the UK population. There are two main funding streams, commissioned and researcher-led.
Commissioned topics are identified through a sophisticated process including scrutiny of systematic reviews and priority setting partnerships with patients, followed by prioritisation processes involving healthcare professionals and patients. In a way, the commissioned arm of the programme can be considered as testing market failures or ’pushing’ the research community to do the sometimes unglamorous research that the NHS needs. This pushing activity is counterbalanced by the HTA researcher-led workstream, where researchers can pitch their own proposals to the programme that are in remit. The creative tension between the programme pushing and pulling research ensures that nothing falls between the gaps. Examples of commissioned and research-led topics are shown in Figure 2.
The HTA Programme also participates in wider NIHR themed calls such as frailty, cannabis-based products for medicinal use, antimicrobial resistance and mental health. Patient and public involvement (PPI) is at the heart of the NIHR and HTA Programme (Figure 1) and is etched into all HTA processes including topic identification, funding and programme oversight. The programme embraces cutting edge methods, and its trials are supported by accredited clinical trial units.
It also supports embedded methodology through its studies within a trial (SWATs), as well as conducting workshops on specific topics such as data-enabled trials. The HTA Programme contracts higher education institutes to deliver the work by rigorous monitoring and it ensures that nearly 100% of the outputs are available in the public domain through the HTA Journal published by the NIHR Journals Library.
Future challenges
The increasingly ageing population with multiple health conditions requires a rethink from researching one disease at a time to researching multimorbidity disease clusters. Some areas of research, such as end of life care and social care are especially challenging. Costs of clinical trials continue to rise, and although efficient data-enabled trials are usually quicker they are not necessarily cheaper. Research following patient need – recruiting from areas of the country where disease burden is greatest is slowly improving, as is ensuring that trial populations represent the communities they are meant to represent in terms of ethnicity and other protected characteristics or inequalities.

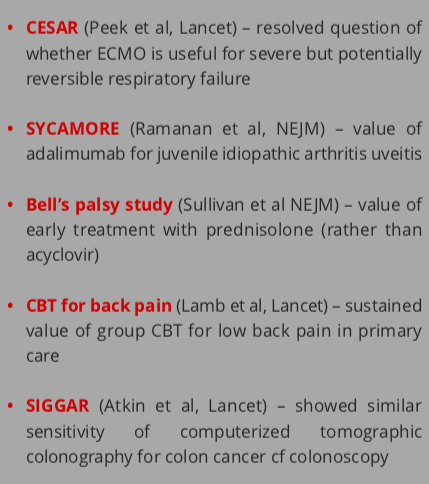
Figure 3. Examples of commissioned NIHR HTA topics (left column) and researcher-led topics (right column)
Reducing research waste has been a prominent theme in the HTA Programme, with special emphasis on reducing duplication and ensuring enhanced dissemination of findings. The threat of emerging infectious disease epidemics is also difficult to research in a reactive fashion, so the HTA Programme has a suite of six ’hibernating’ projects originally designed for the next flu pandemic which can be activated for new threats such as COVID-19. HTA opens its doors to international collaboration since the problems facing many countries are the same. It has successfully funded several joint studies with NHMRC Australia for example.
Transforming people’s lives
Hardly a week goes by without an HTA study appearing in a prestigious journal such as the Lancet or New England Journal of Medicine and outputs are incorporated into guidelines and policies such as those produced by the National Institute for Health and Care Excellence (NICE) and the National Screening Committee. Payback analyses on funds invested in research have consistently demonstrated evidence of knowledge generation, impact on policy and practice with gains for the wider economy. Because health outcomes tend to be better in research-active organisations, it was heartening to observe that in 2019, over 1 million participants were recruited into NIHR studies and 100% of NHS hospital trusts contributed to national studies. The NIHR has effected a culture change in the NHS so that research is now ’everyone’s business’.
Rapid innovation and rising costs are common to all international health care systems, so the need for an independent broker to assess real-world effectiveness and cost-effectiveness has never been greater. The NIHR HTA Programme is efficient, open to innovative methods and adapts to changing health priorities – ‘needs led and science added’.
Contributor Profile
Editor's Recommended Articles
-
Must Read >> Boosting innovation in the NHS
-
Must Read >> £9 million funding for digital health technology