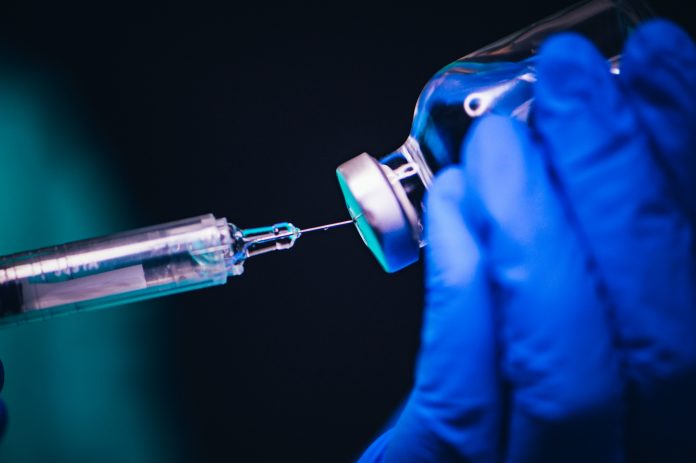
While the US and UK sealed their BioNTech and Pfizer vaccine deals within 24 hours of the new results, the European Commission took three days longer to secure their larger order of 300 million doses
The new agreement between BioNTech, Pfizer and the European Commission will be for 200 million doses – with the option to buy an extra 100 million written in. Having 300 million doses will mean that 150 million people can be vaccinated, which would mean around 20% of the EU’s population of 741.4 million people would be protected against COVID-19.
The clinical study done by BioNTech and Pfizer has 43,538 people, with a success rate of over 90%. This means that nearly 40,000 people are now protected from catching COVID-19 after they took this vaccine. Crucially, 42% of the participants were of diverse racial backgrounds, meaning that the vaccine should theoretically work to protect those who have been shown to be most vulnerable to contracting and dying from the virus.
President of the European Commission, Ursula von der Leyen, said:
“In the wake of Monday’s promising announcement by BioNTech and Pfizer on the prospects for their vaccine, I’m very happy to announce today’s agreement with the European company BioNTech and Pfizer to purchase 300 million doses of the vaccine.
“With this fourth contract we are now consolidating an extremely solid vaccine candidate portfolio, most of them in advanced trials phase. Once authorised, they will be quickly deployed and bring us closer to a sustainable solution of the pandemic.”
Who will get the vaccine first in the EU?
An ethics paper released in Germany suggests that prioritisation should “not be based on medical-epidemiological findings alone. It is rather the case that ethical and legal considerations should play a decisive role, too.”
In the UK, the prioritisation system is going to go from most vulnerable (80 plus years in care homes, plus the workers who look after them) all the way to the final, lowest priority tier of people under 30. A similar system will likely evolve in the EU, especially as the Commission makes moves to build the first European Health Union. This Union is going to be a way to deal more smoothly with cross-border health threats and will reframe existing legal policies that could have hindered the speed of the EU response to COVID-19.
When will Europe receive the Pfizer vaccines?
Currently, deliveries of vaccines by BioNTech and Pfizer are expected to begin in December – the timeline depending on how long the drug takes to go through European Medicine Agency (EMA) approval. BioNTech’s German and Pfizer’s Belgian site will be the ones manufacturing these doses for Europe. Both companies expect to produce 1.3 billion doses in 2021, for global distribution.
What other vaccines have Europe already bought?
The Commission has made a deal with the leading UK vaccine made by AstraZeneca, which enables all EU countries to purchase 300 million doses of the AstraZeneca vaccine, with an option for further 100 million doses. They also have a deal with Sanofi-GSK, which would enable EU countries to buy another 300 million doses – reserved doses can also be donated to lower-and-middle-income (LMIC) countries. Finally, they have a deal with Janssen Pharmaceutica NV, which will enable countries to buy 100 million doses, with the possibility to buy another 100 million.
Altogether, Europe now theoretically has access to 1.2 billion doses of the vaccine, if they all make it successfully through regulation and further clinical trials.