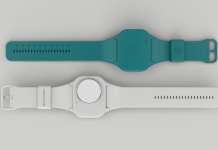
Beatrix Vereijken and Lynn Rochester share how Mobilise-D develops digital technology to measure mobility in daily life to transform clinical research and health
Mobility matters! Mobility, the ability to physically move around, is vitally important and key to our independence. Walking is an important part of mobility – allowing us to carry out daily activities such as getting out of bed, preparing breakfast, and walking to the grocery store. Yet, almost a third of people over 75 have difficulties walking[1], with severe societal and personal consequences[2]. Living with long-term diseases further contributes to the burden of mobility loss. Hence, addressing and reducing mobility loss and its consequences is an important global priority.
Mobility is also an important indicator of health and functional integrity. Even small changes in how we walk, such as how fast or how far, signal underlying problems or worsening health, and provide important insights into multiple health conditions – a sixth vital sign. In conditions where mobility loss is a key feature, such as Parkinson’s disease (PD), multiple sclerosis (MS), chronic obstructive pulmonary disease (COPD), heart failure (CHF), and recovering from a fractured hip (PFF), mobility is an important outcome to measure.
Nevertheless, mobility is not routinely assessed even in trials of new therapeutics[3], partly because we tend to assess mobility one snapshot at a time, leading to imprecise, subjective measures.
Digital health technology, including body-worn devices, can be used to assess mobility, while continuous recording for weeks makes remote monitoring a possibility. Digital mobility assessment can capture discrete, relevant, and ecologically valid mobility outcomes (such as real-world walking speed) that tell us about actual mobility in everyday life, moment by moment changes in mobility and how these relate to health.
This approach also gauges what matters to patients, as well as building inclusivity into the heart of trial design. It is, therefore, no surprise that the pharmaceutical industry wants to use digital mobility outcomes (DMO’s) to accelerate drug development and deliver more efficient, inclusive, and patient-relevant products.
This was the key area of unmet need for industry proposed by Dr Ronenn Roubenoff from Novartis, which led to the formation of the Mobilise-D consortium.
Mobilising efforts! It takes a village…the Mobilise-D Consortium
Using wearable sensor technology to monitor mobility in daily life, Mobilise-D develops and validates the necessary tools to measure real-world mobility outcomes and maps them to various health conditions (PD, MS, COPD, CHF, PFF), with the goal that health authorities and regulators accept the digital assessment of mobility for use in drug development, clinical research and healthcare. An endeavour of this scope requires a strategic multidisciplinary, multi-stakeholder approach in partnership with patients and the public to ensure that all developments are acceptable and fully informed.
Mobilise-D is a five-year public-private partnership launched in April 2019, funded by the European Innovative Medicines Initiative 2 Joint Undertaking[4]. The Mobilise-D consortium (jointly led by Professor Lynn Rochester – Newcastle University, UK and Dr Ronenn Roubenoff – Novartis) includes research partners based at 34 leading universities, pharmaceutical and technical companies[5]. Below are the three grand challenges Mobilise-D are addressing.
Challenge 1 – Can we measure real-world mobility?
Being able to capture mobility changes in people’s everyday life is invaluable to the individual and society alike, but doing so reliably is a tricky problem. A small wearable device coupled with newly developed state-of-the-art algorithms is used to capture digital mobility outcomes continuously at home. To ensure that these have the precision and accuracy required for use in drug development and health, we are conducting a world-first technical validation study on multiple health conditions and healthy people[6].
This allows us to verify device performance, determine the validity and reliability of algorithms in the lab and the real world, and evaluate patient and professional perspectives on the acceptability of the sensors.
Challenge 2 – Are digital mobility outcomes valid and meaningful?
For new digital mobility outcomes measures to be useful, they should provide reliable, accurate measures of mobility, detect changes over time, relate the change to altered health (e.g., worsening of condition) or the effect of an intervention, and capture the change that is meaningful to patients. The Mobilise-D clinical validation study generates the necessary evidence to support clinical validity. We are recruiting >2,000 patients in 16 centres across Europe.
Patients are followed every six months for two years with an extensive clinical assessment and a seven-day digital mobility assessment. The results provide evidence for clinical validity required for regulatory endorsement, as well as unique legacy datasets for future work.
Challenge 3 – Will new digital mobility outcomes be accepted for use in regulatory drug trials and health?
Early and ongoing interaction with regulatory authorities have shaped a regulatory strategy towards the adoption of DMOs[7] and have so far resulted in two letters of support from the EMA[8,9]. Key to regulatory success is our work with patients and the public to develop the concept of mobility measurement as capturing a meaningful aspect of health. Through ongoing interactions with the EMA and FDA, we develop a sound regulatory roadmap towards the approval of novel digital outcomes[10].
Mobilise-D contribution towards sustainable healthcare
The future of sustainable healthcare is digital but requires validated and approved tools. Mobilise-D brings the key ingredients to achieve this. Through publications, state-of-the-art literature reviews[11], sharing data and standards[12], and ongoing public webinars[13], we share our findings to the wider community to help develop harmonised approaches, ongoing learning and uptake of digital mobility assessment.
Additional work with patients and the public has outlined core domains of wider relevance to ensure that developments in digital healthcare are inclusive and acceptable.
Finally, this work requires a new generation of specialist skills in digital healthcare that is fostered by the formation of the Digital Health Catalyst – which provides a space to nurture the professions of the future.
References
- https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe__statistics_on_health_and_disability
- https://www.youtube.com/watch?v=w75iatIQ56I
- https://academic.oup.com/ageing/article/51/1/afab242/6514230
- https://www.imi.europa.eu/projects-results/project-factsheets/mobilise-d
- https://www.mobilise-d.eu/partners
- https://bmjopen.bmj.com/content/11/12/e050785
- https://www.mdpi.com/1424-8220/20/20/5920
- https://www.ema.europa.eu/en/documents/other/letter-support-mobilise-d-digital-mobility-outcomes-monitoring-biomarkers_en.pdf?fbclid=IwAR0bxuPK5P6ZiKe7uE7-Pk00rhUNqHQB3-u7VPRDqTANrQ7PPEbNI6kmEVA
- https://www.ema.europa.eu/en/documents/other/letter-support-mobilise-d-digital-mobility-outcomes-monitoring-biomarkers-follow_en.pdf
- https://www.karger.com/Article/FullText/512513
- https://www.nature.com/articles/s41746-021-00513-5
- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0256541
- https://www.youtube.com/channel/UCZHsEuoUzt1xN4oKNUNbC3A/playlists

Mobilise-D has received funding from the IMI2 JU under grant agreement No. 820820. This JU receives support from the European Union’s Horizon 2020 and EFPIA.
*This is a commercial profile.
© 2019. This work is licensed under CC-BY-NC-ND.