Cecilia Van Cauwenberghe from Frost & Sullivan’s TechCasting Group, walks us through multiple sclerosis facts & trends
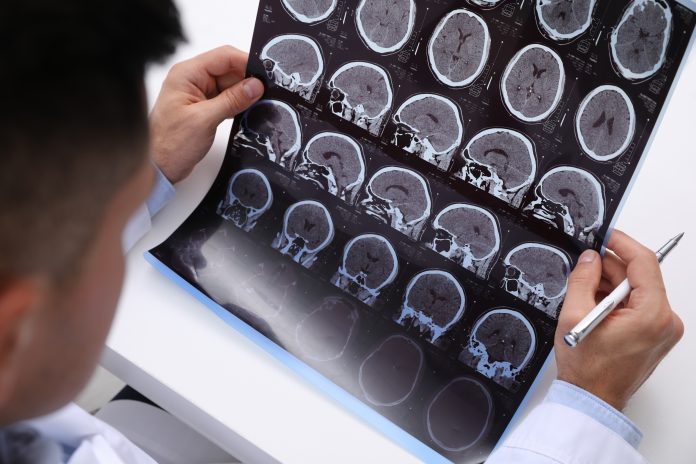
Multiple sclerosis (MS) is one of the most prevalent central nervous system (CNS) disorders which rapidly progresses with age. MS is an autoimmune condition where the myelin sheath of neuronal, spinal, and optic nerves is damaged, resulting in several disabilities such as fatigue and reduced eyesight. Multiple sclerosis facts & trends demonstrate how MS reduces the brain and cognitive reserve and delays the appearance of age[1]related neurodegenerative disorders in later life (Sengupta, 2017).
According to the Multiple Sclerosis International Federation (MSIF), the most common type of MS is relapsing-remitting MS (RRMS). Around 85% of MS patients are diagnosed with RRMS. The MSIF reports that MS affects over 2.8 million people worldwide. Near 25% of them residing in low- and middle-income countries. Women are twice as likely to have MS than men; although, in certain countries, the female to male MS ratio can be 3:1.
Disease-modifying therapies (DMTs) for people with MS are partially or wholly government-funded in high[1]income economies, whereas there is no such federal support in low-income countries. Disparities per country and region in the availability of treatment for MS are notable. In Africa, for instance, 60% of countries have no licenced DMTs, and this number is even higher, 70% when analysing low-income countries around the world (Piggot et al., 2021).
The researchers emphasise the need for guidance for off-label treatments as a critical matter. Off-label DMTs are sometimes more available or affordable (Laurson-Doube et al., 2021). The Atlas of MS highlights that at least 89 countries use one off-label DMT to treat MS (Walton et al., 2020).
Main therapies and trends
The treatment of MS comprises disease-modifying therapies (DMTs) that are highly MS-specific and symptomatic therapies (STs) that are more generic and usually used in different disease areas to treat common symptoms resulting from neurological dysfunction (Dobson and Giovannini, 2019).
As the number and efficacy of DMTs increase, the main focus is on early diagnostics of MS to prevent long[1]term disability, a focus that has grown. Early treatment of those at risk of long-term disability is needed to minimise the physical morbidity associated with MS. Treatments consist of immunosuppressants (e.g., fingolimod, natalizumab, ocrelizumab), or immuno[1]modulatory agents (e.g., interferon beta, glatiramer acetate, teriflunomide).
There are several new treatments in the pipeline for MS. However, current disease-modifying treatments are only available to people with clinically relapsing forms of the disease and a minority of those with progressive disease (Dobson and Giovannini, 2019).
The role of digital healthcare
Researchers working at the Multiple Sclerosis Center Dresden, Dresden University of Technology, have reviewed the last innovations in digital solutions for people living with MS (Ziemssen and Haase, 2021). According to the researchers, facing a disease onset in their early adulthood is often seen as the ideal target group for new trends in digital healthcare because of their demand for a more personalised and tailor-made disease management. This fact is particularly relevant due to the complexity and heterogeneity of MS.
The development of digital applications and remote communication technologies for people with MS has increased notably. Indeed, eHealth apps have improved outcomes and facilitated access to care, disease information, and support. From the perspective of healthcare professionals treating patients with MS, such eHealth technologies facilitate clinical disability assessment.
Even other clinical practices, including laboratory analysis, imaging technologies and remote monitoring, have been enhanced thanks to digital healthcare applications. The patient’s symptoms, adverse events, and outcomes can be tracked more precisely, hence allowing optimising time and intervention quality.
Acknowledgements I want to thank all contributors from the industry involved with developing and delivering this article from Frost & Sullivan.
Further reading
- Dobson, R. and Giovannoni, G., 2019. Multiple sclerosis–a review. European journal of neurology, 26(1), pp.27-40.
- Laurson-Doube, J., Rijke, N., Helme, A., Baneke, P., Banwell, B., Viswanathan, S., Hemmer, B. and Yamout, B., 2021. Ethical use of off-label disease-modifying therapies for multiple sclerosis. Multiple Sclerosis Journal, 27(9), pp.1403-1410.
- Piggott, T., Nonino, F., Baldin, E., Filippini, G., Rijke, N., Schünemann, H. and Laurson-Doube, J., 2021. Multiple Sclerosis International Federation guideline methodology for off-label treatments for multiple sclerosis. Multiple Sclerosis Journal–Experimental, Translational and Clinical, 7(4), p.20552173211051855.
- Sengupta, D., 2017. Therapeutic Breakthroughs in Multiple Sclerosis – Future Tech TechVision Opportunity Engine Disease Modifying Therapies Lead the MS Landscape, TechVision Opportunity Engines, D835-1C.
- Walton, C., King, R., Rechtman, L., Kaye, W., Leray, E., Marrie, R.A., Robertson, N., La Rocca, N., Uitdehaag, B., van der Mei, I. and Wallin, M., 2020. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Multiple Sclerosis Journal, 26(14), pp.1816-1821.
- Ziemssen, T. and Haase, R., 2021. Digital Innovation in Multiple Sclerosis Management. Brain Sciences, 12(1), p.40