Medicinal chemists describe how small molecule probes allow for the detection of CB2R, and thereby enable the discovery of novel anti-inflammatory treatments
The G-protein-coupled receptor (GPCR) CB2R (1) is an essential constituent of the endocannabinoid system (ECS), a central lipid signalling system in all vertebrates (2). Preclinical studies have shown that CB2R activation is a general and robust principle to attenuate inflammation and associated tissue injury in a wide variety of pathological conditions.
Strong accumulating evidence suggests that diseases such as heart disorders, gastrointestinal, liver, kidney, lung, neurodegenerative or neuroinflammatory diseases, pain, skin pathologies, rheumatoid arthritis, endometriosis and eye diseases might be therapeutically addressable by CB2R (3).
Type-2 Cannabinoid Receptor (CB2R) – A key toward the treatment of inflammatory diseases
Although several promising therapeutic small molecule drugs are in advanced clinical trials, no selective CB2R activators, so-called agonists, have reached the market to leverage the enormous therapeutic potential of CB2R. The reason for this is challenges related to:
- Determining the localization and functional expression of CB2R;
- Insufficient quality and selectivity of early tool compounds;
- Overall characteristics of first drug development candidates;
- Lack of translatability of promising preclinical efficacy data toward humans which has been discussed in a recent open access government contribution entitled ‘Challenges bringing CB2R medicine to bedside’(4).
Tools for detecting CB2R protein
While sophisticated mRNA techniques reliably enable the measurement of CB2R messenger RNA (mRNA) expression, the functional detection and localization of CB2R protein remained challenging for the past three decades.
Generally, antibodies are used for such purposes but in the case of CB2R no specific antibodies could be obtained despite extensive investigations. Only very recently, highly selective chemical tools, so-called labelled chemical probes, have been developed which now allow for the reliable detection of CB2R protein (5).
A labelled chemical probe is a small molecule that is a specific ligand for the target protein of interest, in our case CB2R, and bears a reporter unit that allows a spatiotemporal characterization of target ligand-protein interactions. Such probes can be used to verify e.g., target protein engagement and quantify target occupancy, identify biomarkers and thereby support preclinical studies and human trials with therapeutic CB2R agonists (Figure 1).
While antibodies usually are limited to the detection of one animal species and require considerable effort and experimental adaptions for intracellular delivery or in vivo applications, a particular advantage is that a labelled chemical probe can be derived from small molecule ligands similar to the present drug molecule and can therefore be used throughout the entire translational path across species. Particularly fluorescently labelled and positron emission tomography (PET) tracers are of highest relevance as chemical probes for CB2R.
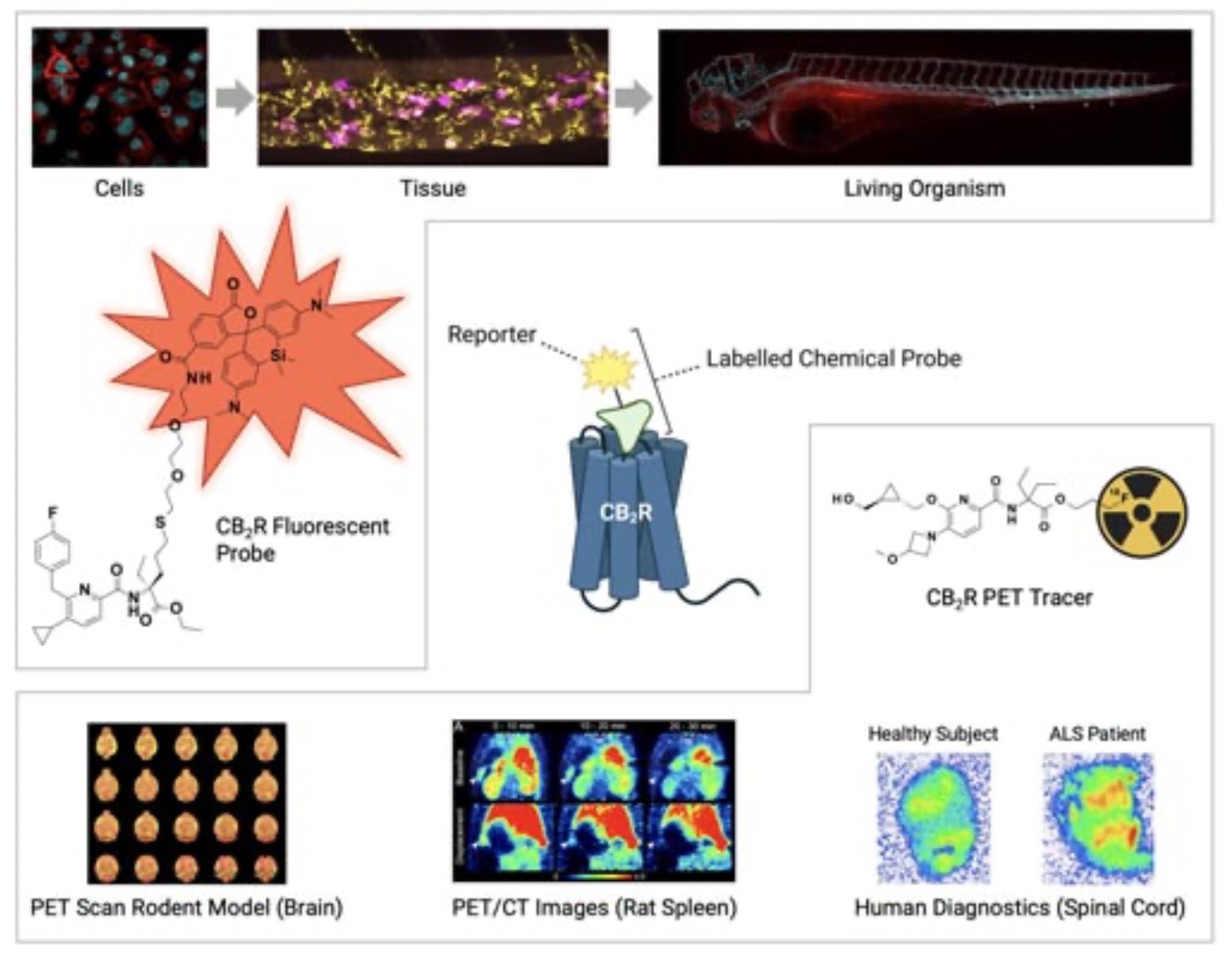
Fluorescently labelled CB2R ligands – Illuminating CB2R
Fluorescently labelled CB2R probes carry a fluorescent dye serving as a light-based reporter group. When a specific wavelength of light is shone on them, the dye absorbs the light and then re-emits a different specific wavelength. This emitted light can then be detected and analyzed in colorful images up to single molecule resolution. Because the fluorescent probes are attached to CB2R, the fluorescence signal detected indicates the location of that receptor.
Highly specific novel CB2R fluorescent probes have been successfully applied to detect and visualize the cellular trafficking of CB2R in different living cellular systems and were used to study cell signalling mechanisms and expression throughout the body in living organisms (6). In clinical studies, such probes can be applied for detecting target occupancy levels of a development candidate e.g. in blood samples from healthy volunteers or patients thus supporting the identification of therapeutically relevant doses for anti-inflammatory treatments.
CB2R PET tracers – Non- invasive 3D visualization of CB2R in living organisms
PET tracers belong to the group of radiolabeled imaging agents. The introduction of the PET emitting atom is of particular advantage since depending on the used isotope there is no or only minimal one single atom changes for the introduction of the reporter necessary.
Therefore, no alteration of the pharmacological properties of the parent drug is observed.
Such chemical probes are applied for disease quantification, e.g. by comparing protein distribution and levels in the healthy and diseased state, for drug candidate selection and for facilitating their clinical development toward anti-inflammatory treatments.
A prime area of application is the therapeutic dose selection in clinics, which is decisive for successful enrollment of the clinical trial and challenging when like for CB2R no predictive animal model or validated clinical biomarker is available – especially for targets in the central nervous system or organs that cannot be sampled easily. For these drugs, imaging agents are the only way to quantify target engagement in patients and predict a clinical dose that will achieve efficacy with minimal unwanted side effects in anti-inflammatory treatments.
Recently discovered CB2R PET tracer [18F]RoSMA-18-d6 is based on the short-lived positron emitter [18F] (7). It was successfully applied in vivo PET studies and allowed to detect CB2R upregulation on post-mortem human ALS spinal cord tissues thus holding great promise for using CB2R as a marker of neuroinflammation and for supporting clinical studies with therapeutic CB2R agonists for the treatment of neuroinflammatory diseases.
In summary, the recently discovered high-quality labelled CB2R fluorescent and PET probes have significantly contributed to a better understanding of CB2R expression in health and disease states across species and deepened the insights into the receptors function and mechanism of action.
Furthermore, these probes allow translational studies to an unprecedented extent supporting the clinical development of CB2R agonists and ultimately unlocking the great potential of this receptor for anti-inflammatory treatments.
References
- a) Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61-65; b) Griffin G, Tao Q, Abood ME (2000) Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 292: 886-894; c) Maccarrone M, Grether U (2022) Is CB2R a hidden treasure trove for treating inflammatory diseases? Open Access Government.
- Maccarrone M (2020) Missing Pieces to the Endocannabinoid Puzzle. Trends Mol Med 26(3): 263-272.
- a) Pacher P, Mechoulam R (2011) Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50(2): 193–211; b) Gasperi V, Guzzo T, Topai A et al. (2022) Recent Advances on Type-2 Cannabinoid (CB2) Receptor Agonists and Their Therapeutic Potential. Curr Med Chem https://doi.org/ 10.2174/0929867329666220825161603.
- Pacher P, Grether U (2023) Challenges bringing CB2R medicine to bedside. Open Access Government.
- Guberman M, Kosar M, Omran A et al. (2022) Reverse Design toward Optimized Labeled Chemical Probes – Examples from the Endocannabinoid System. Chimia 76(5): 425-434.
- a) Gazzi T, Brennecke B, Atz K et al. (2022) Detection of cannabinoid receptor type 2 in native cells and zebrafish with a highly potent, cell-permeable fluorescent probe. Chem Sci 13: 5539-5545; b) Sarott RC, Westphal MV, Pfaff P et al. (2020) Development of High-Specificity Fluorescent Probes to Enable Cannabinoid Type 2 Receptor Studies in Living Cells. J Am Chem Soc 142: 16953-16964.
- Haider A, Gobbi L, Kretz J et al. (2020) Identification and Preclinical Development of a 2,5,6-Trisubstituted Fluorinated Pyridine Derivative as a Radioligand for the Positron Emission Tomography Imaging of Cannabinoid Type 2 Receptors. J Med Chem 63: 10287-10306.
Acknowledgements
We wish to thank all colleagues who have contributed over the years to our investigations on CB2R.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


