Henri Huttunen, Chief Scientific Officer, Herantis Pharma Plc, charts progress in the development of disease-modifying treatments for Parkinson’s disease
Recent FDA approvals of two potentially disease-modifying Alzheimer’s disease drugs give hope that similar successes in Parkinson’s disease may be around the corner. However, despite the recent advances in understanding complex disease biology and the development of novel drug targets and biomarkers, progress in the clinical development of new treatments remains slow.
The need for disease-modifying treatments to improve quality of life
James Parkinson published his essay ’Shaking Palsy’ in 1817, describing the clinical features of Parkinson’s disease (McDonald, 2018). In the late 1950s, the discovery of dopamine as the key neurotransmitter regulating movement laid the foundation on how the symptoms of Parkinson’s are treated still today. Nevertheless, the biggest challenge – how to stop or delay the progression of the disease – remains unaddressed. As a result, nearly 10 million people with Parkinson’s disease need disease-modifying treatments to improve their quality of life and, importantly, give them hope and a chance for healthier aging.
During the past decades, we have seen a surge in research interest, public funding and clinical trials to expedite the development of treatments that address the underlying pathological mechanisms of neurodegeneration and would stop the disease progression (disease modification). As the most common form of dementia, Alzheimer’s disease has led the way, but Parkinson’s, the second most common neurodegenerative disease, is not far behind.
2022 was an important landmark in Alzheimer’s disease with FDA approvals for two amyloid immunotherapies, aducanumab and lecanemab by Biogen/Eisai (Lancet, 2022). These passive immunotherapies are the first approved treatments for Alzheimer’s that are considered disease-modifying and are based on the idea that antibodies binding to specific pathological forms of aggregated beta-amyloid protein in the brain can slow down disease progression. Pathological protein aggregation in the brain is a shared feature with several neurodegenerative diseases (Wilson, 2023).
In Parkinson’s, Lewy bodies, clumps of aggregated alpha-synuclein protein are a typical neuropathological feature. Like the recent FDA-approved Alzheimer’s drugs, several alpha-synuclein immunotherapies are in clinical development for Parkinson’s (Knecht, 2022).
Why is progress in the development of new disease-modifying treatments for neurodegenerative diseases so slow?
It took about 20 years to bring amyloid immunotherapies to market approval in Alzheimer’s, and the standard of care for Parkinson’s disease is based on a 60-year-old treatment concept. So why is progress in developing new treatments for ageing-related brain diseases so slow?
First of all, the underlying pathology at the genetic, molecular, cellular, and tissue levels has turned out to be very complex and heterogenous (Jankovic, 2020, Panicker, 2021, Gaertner, 2022). This results in clinical heterogeneity and the disease progresses at different rates in different patients. Instead of a one-size-fits-all strategy, it seems that Parkinson’s disease, should be considered an etiologically heterogeneous syndrome rather than a single disease. This has several fundamental implications for all aspects of drug development, from animal disease models to biomarker development and clinical trial designs.
Nevertheless, despite the diversity of the potential underlying genetic or environmental triggers of the disease, it appears that there are some common features shared by most subtypes of disease, such as accumulation of protein aggregates, altered energy metabolism and waste clearance, and chronic inflammation in the brain (Calabresi, 2023, Tansey, 2022). Finally, clinical trials progress in stages, and due to the slow progression of the disease, an individual clinical trial may require years to demonstrate meaningful changes in the progression of the disease.
Biomarkers help to understand complex disease biology & to stratify patients
Genetic studies of Parkinson’s have identified many causative and risk genes that together have indicated a group of cellular processes to be centrally involved in the pathobiology of the disease. Novel drug targets have also emerged. While there is still plenty of work to do even with the basic research questions, we can now tailor specific treatments for some disease subtypes, such as the LRRK2 inhibitors by Denali/Biogen (Jennings, 2023).
The learnings from the fields of Alzheimer’s and oncology have shown that biomarkers are a paramount part of clinical trial success. Biomarkers based on brain imaging, fluid biopsies, or genetics can be used to stratify patients based on the subtype of disease they have and monitor how the body is responding to the treatment.
Moreover, as a movement disorder, Parkinson’s offers fascinating novel technological approaches for continuous objective measurement of patients’ symptoms with smartwatches, offering new ways to improve the collection, analysis and granularity of data in clinical trials (Lipsmeier, 2022, Sieberts, 2021, Stephenson, 2021).
Compared to traditional clinical metric-based designs, biomarkers can make a difference between failure and success in demonstrating treatment efficacy in a clinical trial.
Is disease heterogeneity a friend or foe?
The personalized medicine ethos is attractive for clinical development and applies well to some of the known genetically defined subtypes of Parkinson’s disease. However, the challenge is that for the vast majority of Parkinson’s patients, the disease appears to be idiopathic, i.e., without a known cause.
Immunotherapies that boost the clearance of protein clutter from the brain have shown that targeting a common disease feature could be one successful strategy. However, will ‘improved waste management’ be sufficient to bring the patients a clinically meaningful, long-lasting improvement of the cardinal symptoms of the disease? Are there better ways, or are there ways that could be combined with immunotherapies to achieve stronger treatment effects?
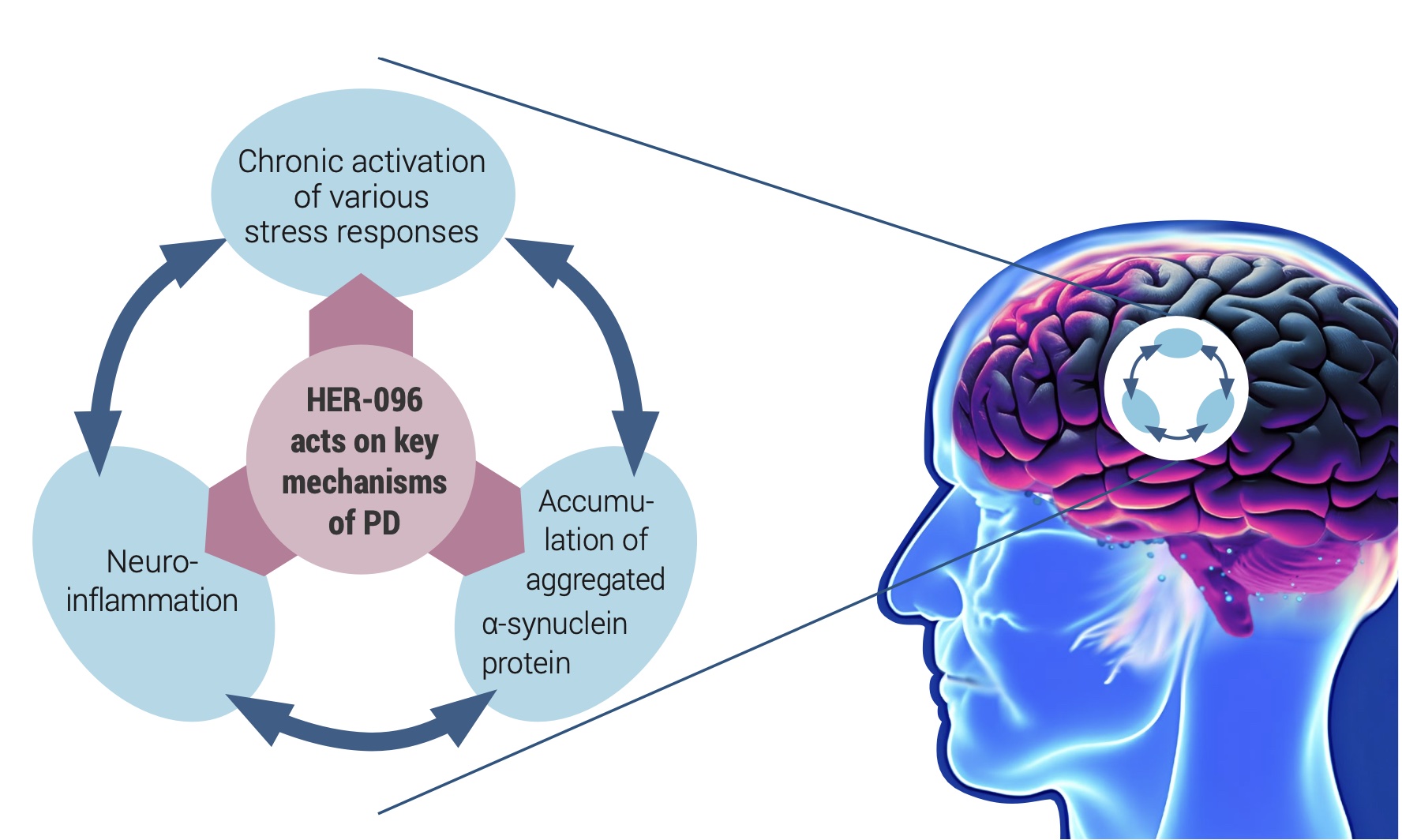
Herantis Pharma, a Finnish clinical-stage biotech company, is developing a novel disease-modifying treatment for Parkinson’s disease. Herantis’ lead product HER-096 mimics our body’s built-in mechanism for maintaining the health and functionality of dopamine neurons by counteracting the adverse effects of the alpha-synuclein aggregation and modulating the inflammatory responses in the brain tissue.
Preclinical studies with HER-096 and its parent protein CDNF (Lindholm, 2022) have shown improved proteostasis and reduced inflammation in association with restored neuronal functionality. After promising preclinical data, HER- 096 is being tested in a first-in-human clinical study. Topline data from this healthy volunteer Phase 1a trial is expected at the end of 2023.
There is an enormous public health need for effective disease-modifying treatments to stop the progression of Parkinson’s disease. Learnings from past clinical trials that help to improve trial designs with targeted patient selection and more sensitive biomarkers will hopefully pave the way to success for companies like Herantis Pharma in their mission to improve the lives of millions of patients.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.