DeepMed has developed an AI oncology diagnostics system, DeepPathTM – LYDIA, to assist histopathologists in performing lymph node metastasis detection quickly and accurately
DeepMed is an ISO-13485 certified start-up MedTech company based in Manchester, UK. Through the support of SBRI we developed an AI-powered product, DeepPath™ – LYDIA, to assist histopathologists in performing lymph node metastasis detection quickly and accurately.
DeepPath™ – LYDIA is expected to improve and accelerate the oncology diagnosis and treatment pipeline, which is currently under pressure. The system was CE-Marked in May 2022 as a Class I decision support product as per IVDD.
AI oncology diagnostics can support overstretched pathologists
The oncology-histopathology combination was selected as our initial focus for two reasons. Cancer incidence is growing globally as the population ages, and histopathology is at the centre of all diagnostics.
In addition, histopathology itself drives patient management and affects the outcome of patient treatment. Secondly, the field of histopathology needs more experts.
According to the Royal College of Pathologists workforce survey (2018), pathology requests grow at 4.5% p.a., while staff numbers grow by only 1.2%. In 2018, 97% of pathology departments in the UK reported inadequate staffing.
The USA reports state a 20% decrease in active pathologists in the past decade. That shortage of pathologists causes pathology departments to be under intense pressure, resulting in a 15% drop in diagnostic accuracy, long waiting times to receive results, and up to two months of treatment delays.
All these issues directly affect patient outcomes, overall survival, and quality of life.
How does the AI oncology diagnostics system, DeepPath™ – LYDIA, work?
Histopathological diagnosis in cancer consists of two main pillars: grading and staging.
In grading a tumour, a tumorous tissue section is examined under a microscope, and based on the morphological characteristics of the whole tumour and the tumour cells, the cancer type and subtype are deduced. This indicates the tumour’s aggressiveness and guides the oncologists towards specific therapeutic strategies.
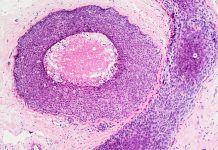
On the other hand, in staging, tissue sections from lymph nodes surrounding the tumour site are examined under a microscope to determine whether the tumour has spread beyond the primary site, an event known as metastasis.
The oncologists completely alter the consequent diagnostic procedures and therapeutic protocols if a tumour has metastasised. DeepPath™ – LYDIA focuses on the former diagnostic aspect, namely staging, to offer decision support to histopathologists.
DeepPath™ – LYDIA is a groundbreaking AI oncology diagnostics system that detects metastatic tumours on digitised microscope slides of Hematoxylin & Eosin (H&E) stained lymph nodes.
It is the first CE-Marked metastasis detection system to identify tumours from four different cancer types: breast, lung, colon and melanoma, accounting for ~43% of total cancer incidence.
The AI oncology diagnostics system outlines the detected tumour regions (also measuring maximum diameter for each region) and presents them to the histopathologist for making the diagnosis; it also re-ranks multiple lymph node slides that belong to a single case from high to low or no tumour content for a further efficiency boost.
The system is compatible with the most popular Whole Slide Image scanners (used to digitise microscope slides).
Furthermore, it makes sure that the histopathologist does not miss any tumour sites with a sensitivity of >97%, while at the same time, it does not clutter the screen with false positives (specificity >90%).
The system is flexible since it can be deployed on the cloud or locally. Furthermore, it can be offered as a standalone system. However, the preferred way to maximise workflow efficiency is as a plug-in to the Picture Archiving and Communication System (PACS) already used by each lab.
We have already integrated DeepPath™ – LYDIA into the SECTRA PACS for digital pathology, which is one of the leading PACS solutions and we continue integrating into other PACS systems as driven by market demand.
The system currently undergoes pilot testing in hospitals across the UK and EU. Furthermore, we are actively working towards expanding DeepPath – LYDIA coverage to more tumour types to deliver the clinic’s first pan-cancer metastasis detection system for cancer staging worldwide.
Beyond Diagnostic Decision Support
DeepMed participated in the public VisioMel-Challenge along with 540 other participants and, through just three submissions, made it to the top 10 with a performance that is marginally close to the leader.
This competition concentrated on predicting relapse in melanoma patients from digitised slides of H&E-stained biopsies using AI. The competition was based on the extensive RIC-Mel dataset, which included data from over 40,000 patients across 49 sites in France.
VisioMel was a collaborative effort initiated in 2021 by the French Society of Pathology, the French Society of Dermatology, the French Cutaneous Cancers Group, and the National Professional Council of Pathologists.
The primary sponsor was the French Ministerial Delegation for Digital Health as part of the French government’s ‘Digital Health Acceleration Strategy’.
DeepMed intends to use its technology, methodology and know-how to develop AI-powered modalities capable of augmenting or even replacing expensive and time-consuming molecular tests for relapse prediction in oncology, such as DecisionDx- Melanoma, Oncotype DX for breast cancer, Oncotype DX Genomic Prostate Score and others.
Those modalities, being much cheaper and having far-less turn-around time, will enable the democratisation at a global scale of predictive and personalised medicine.
As such, apart from the UK, Europe and the US, healthcare systems in developing countries unable to sustain the current technology would now have access to much cheaper and faster alternatives.
This would enable them to offer higher quality services, which would translate to prolonged survival and higher quality of life for patients, constituting our sole mission.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.