Dr Carlos Ziebert, Leader of the Group Batteries – Calorimetry and Safety, KIT, explains how generated heat and self-discharge of magnesium batteries can be studied through calorimetry
In 2019, the KIT, the University of Ulm, the Center for Solar Energy and Hydrogen Research Baden- Württemberg, and the University of Giessen jointly launched the POLiS – Cluster of Excellence for Battery Research Post Lithium Storage, funded by €47 million over seven years.
Such post-lithium batteries use more abundant and environmentally friendly materials, such as Sodium, Magnesium or Calcium, instead of Lithium, Nickel and Cobalt. The work in the group Batteries – Calorimetry and Safety at the Institute for Applied Materials – Applied Materials Physics (IAM-AWP) started with coin cells that were provided by the Helmholtz Institute Ulm (HIU) and the Institute of Nanotechnology (INT).
Electrochemical test
In these cells 14-polyanthra-quinone (14PAQ) cathodes were assembled against Mg-foil as an anode by using 0.3 M magnesium tetrakis (hexafluoroisopropyloxy) borate Mg[B(hfip)4]2/dimethoxy-ethane (DME), 0.5 M Mg[B(hfip)4]2/DME and 0.5 M Mg[B(hfip)4]2/tetraglyme (G4) electrolytes.
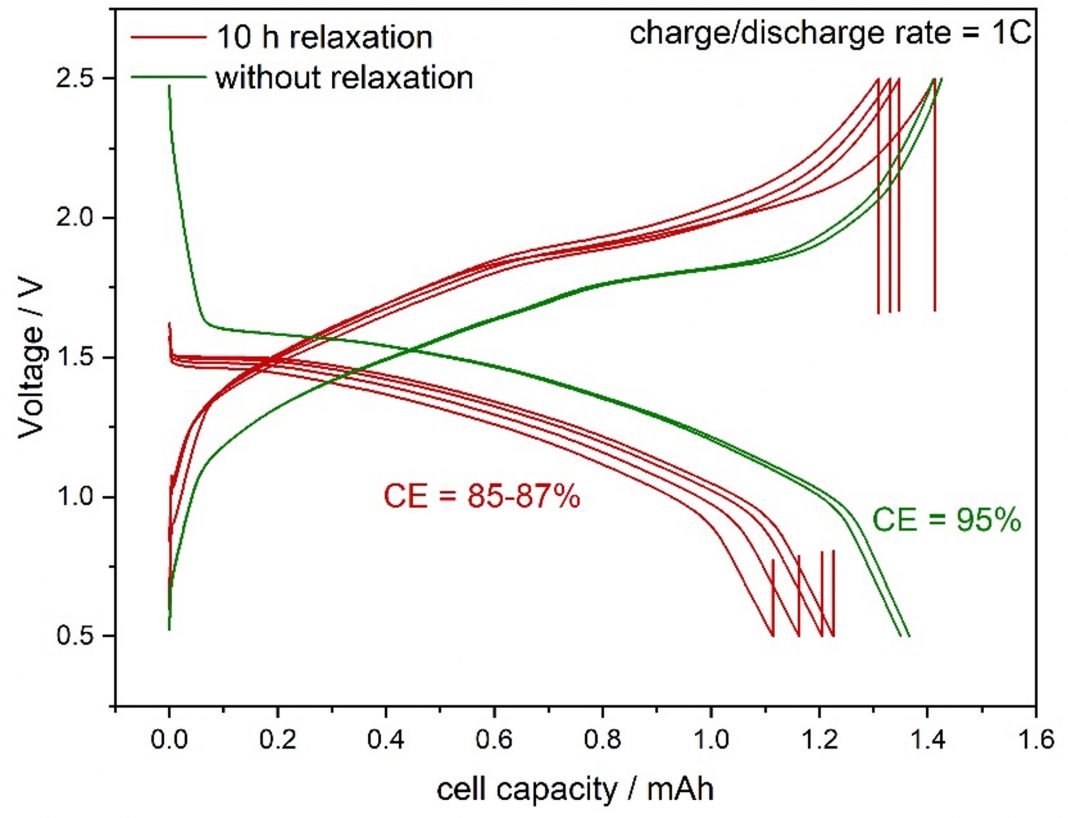
The MS80 Tian-Calvet calorimeter allows us to determine both the generated heat during cell operation and the self-discharge in the relaxation periods of these cells. Fig. 1 compares the voltage vs capacity curves at 1.0 C charge/discharge rate without (green curve) and with 10 h relaxation (red curve). It can be clearly seen that the relaxation leads to a 10% reduction both in capacity and in Coulombic Efficiency (CE). The latter is defined as the ratio between the charge and the discharge capacity. This level of reduction indicates self-discharge.
Self-discharge test
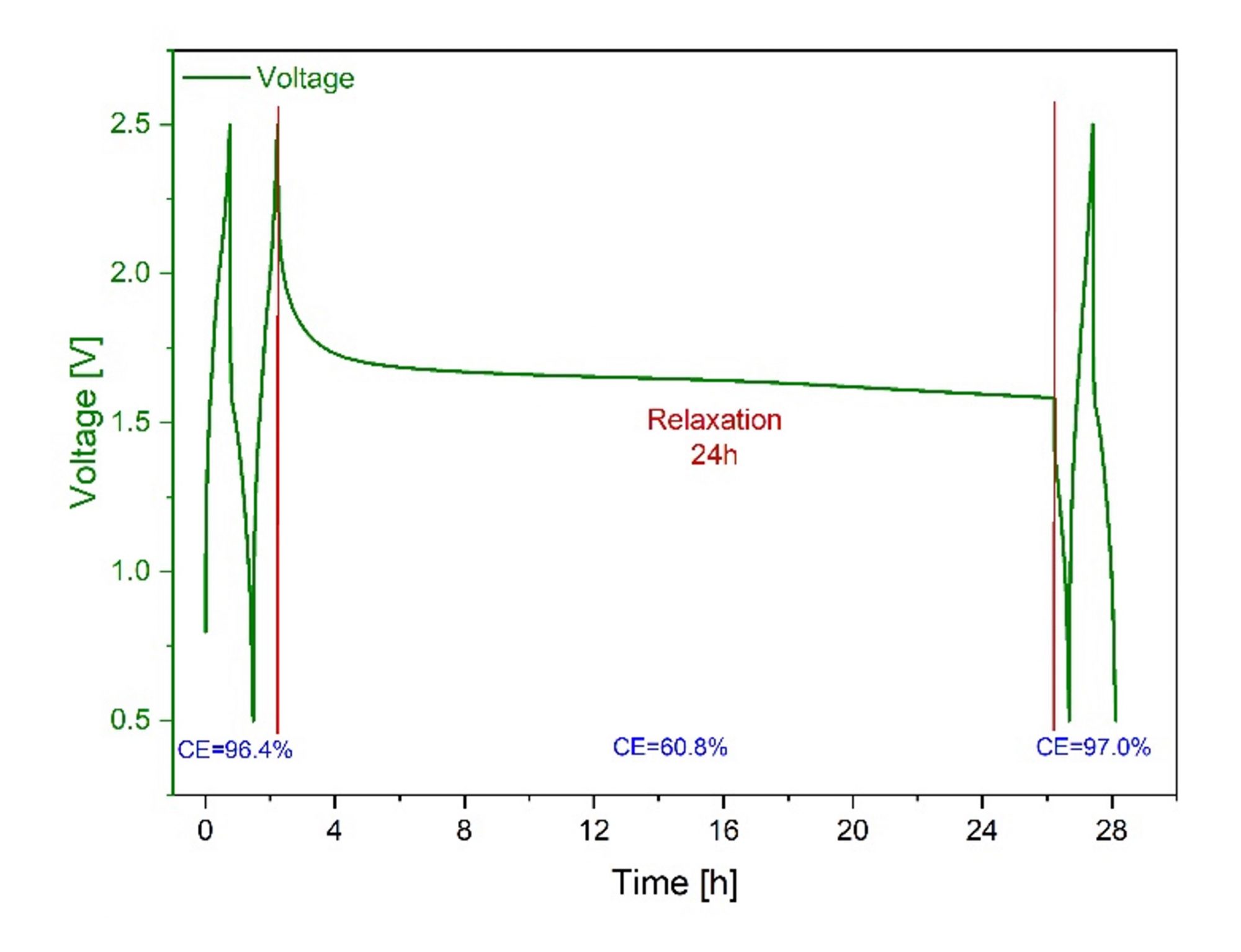
Undesired parasitic chemical reactions lead to a spontaneous and irreversible capacity reduction by self-discharge without any external electrical connection in the Mg coin cells. This becomes even more pronounced in the 24h self-discharge test shown in Fig. 2.
In this test, the coin cells are charged and discharged in the MS80 calorimeter with 1.0 C for two cycles and then held for 24 h at the fully charged state. Then the cells are discharged with the same C-rate, and finally, they are charged and discharged again for two cycles to determine the change in CE. A high level of self- discharge of 36% was found given in terms of Coulombic efficiency.
Determination of generated heat by calorimetry
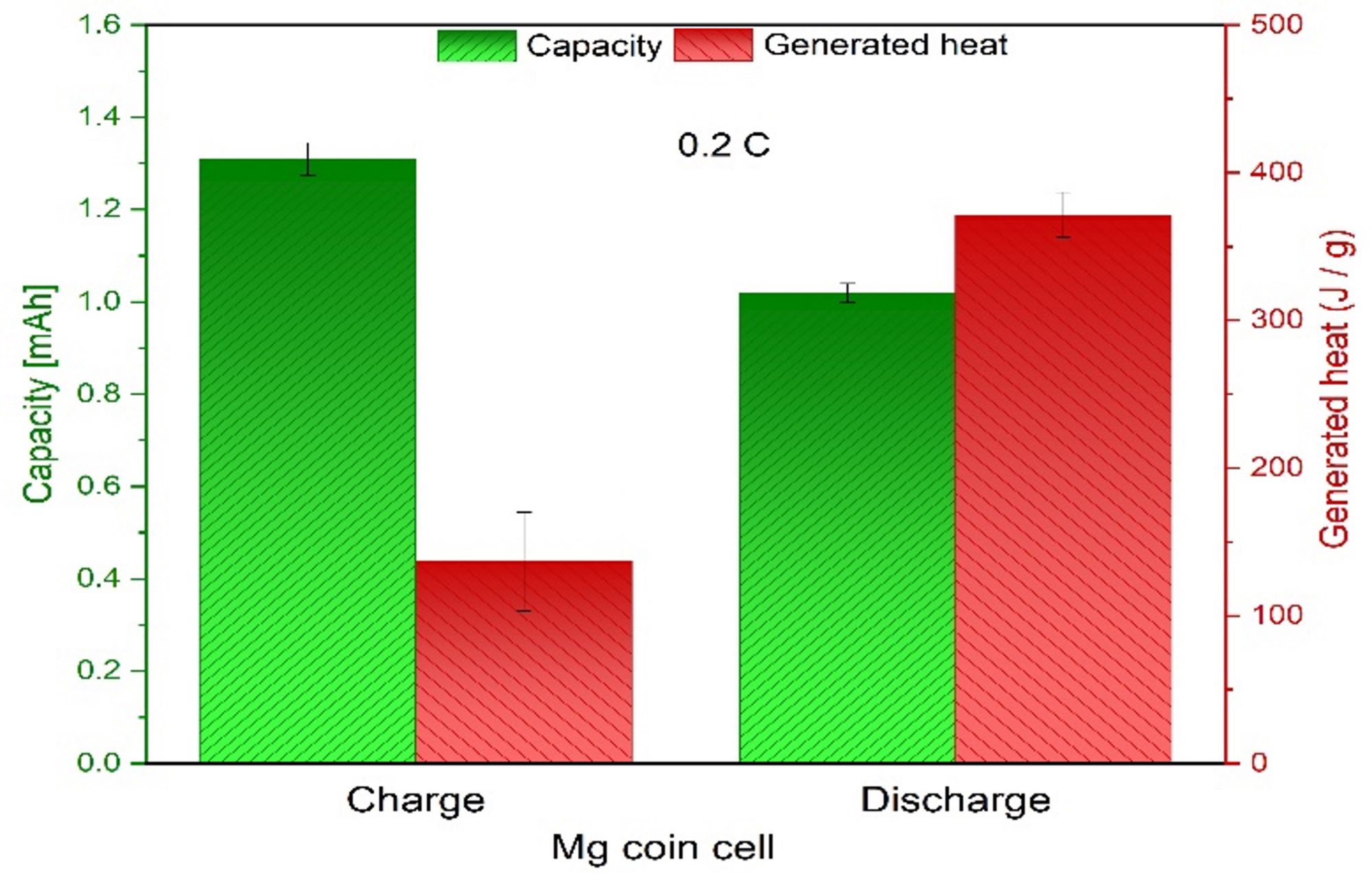
The total heat generation can be accurately determined during cycling by the direct heat flow measurement capability of the MS80 calorimeter. Fig. 3 shows the capacity and the generated heat per mass (J/g) that has been determined by integration over the heat flow curves at 25°C for a 0.2 C rate. The generated heat amounts to 325 J/g during charging and 375 J/g during discharging. For a 1.0 C rate, these values are a little bit higher with 375 J/g and 450 J/g.
Interestingly, the heat flow in these organic-based 14PAQ cells is negative during charging. This indicates that the cell absorbs heat (endothermic reaction) during the magnesium extraction. This can be attributed to entropy change during de-magnetisation, and entropy changes are responsible for the heat absorption associated with material phase changes in the cell.
Thus, it has been demonstrated that calorimetry gives insights into the underlying reaction mechanisms and heat conduction processes in Mg batteries. The next steps will be material optimization to reduce the self-discharge, followed by safety tests and upscaling to the pouch cell scale.
www.postlithiumstorage.org/en/
The work described here contributes to the research undertaken by CELEST (Center for Electrochemical Energy Storage Ulm-Karlsruhe). The German Research Foundation (DFG) under Project ID 390874152 (POLiS Cluster of Excellence) funds this work

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.