Professor Mark Cunningham and Dr Kate Connor from Trinity College Dublin discuss the burden of brain tumour-related epilepsy and why novel therapies are urgently needed to improve the quality of life for those affected
Brain tumour-related epilepsy (BTRE) is a unique and devastating condition which embraces two serious pathologies. BTRE incidence is inversely correlated to malignancy, with seizures as the first presenting symptom in 65-85% of patients with low-grade gliomas (LGG) and 30-60% with glioblastoma (GBM). (1)
Seizures often manifest as complications throughout brain tumour evolution. Clinical management via radiotherapy, chemotherapy, and antiepileptic drugs (AEDs) can reduce seizure frequency, but many patients continue to experience seizures following therapeutic intervention. (2) Overall, BTRE is a debilitating and difficult-to-control condition, with seizures closely linked to tumour recurrence and progression.
In all cases, seizures associated with brain tumours significantly impact patients’ lives. BTRE is a major risk factor for long-term disability in patients due to the negative impact of seizures on quality of life (QoL) and the side effects of prescribed AEDs. (3) Patients with seizures report reduced physical and mental health, increasing seizure burden and anticonvulsant use associated with reduced cognitive performance. (4)
Seizures result in reduced mobility (loss of driving license), contribute to behavioural issues, and increase the risk of death (via uncontrollable seizures). Critically, given the limited efficacy of surgical, oncological, and pharmacological treatments for BTRE, and its significant impact on quality of life, there is an urgent need to expedite the development of novel therapies.
Glutamatergic mechanisms in brain tumour-related epilepsy
Epilepsy is fundamentally characterised by aberrant neuronal excitability. Notably, glutamate (the major excitatory neurotransmitter in the central nervous system) within the peritumoural microenvironment is a key contributor to malignant tumour growth and seizure generation. (5)
Specifically, abnormal glutamate expression (i.e., elevated extracellular levels of glutamate and excessive glutamate receptor activation) leads to uncontrolled neuronal excitability, promoting synchronous neuronal epileptic discharges. (6) Glutamate induces calcium-dependent depolarisation of post-synaptic neurons, causing excitotoxicity and subsequent neuronal cell death. (7) This dynamic and destructive process promotes tumour growth and glioma cell infiltration into the surrounding brain parenchyma. The critical role glutamate plays in glioma growth and epileptogenesis highlights the glutamate system as a powerful therapeutic target (8) and biomarker (9) for BTRE treatment.
Innovative therapeutic approaches for BTRE
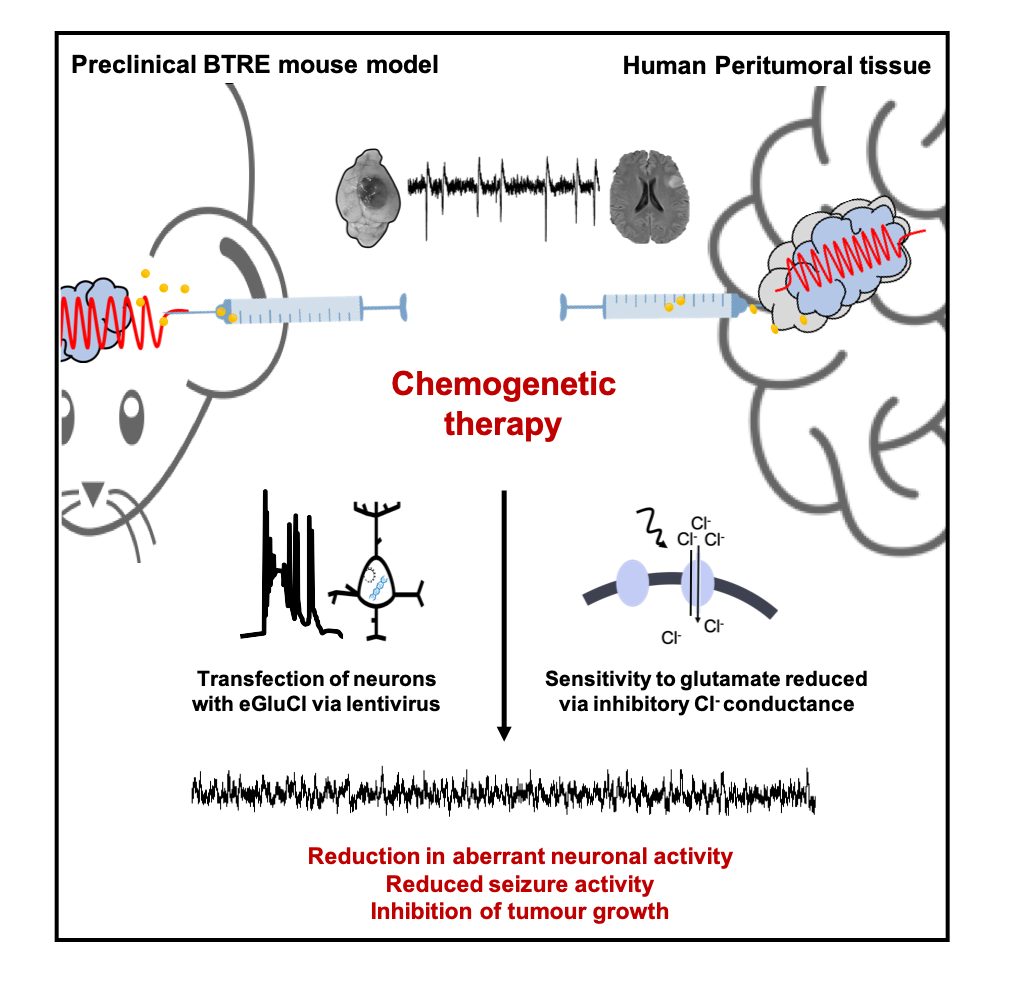
To date, attempts to treat BTRE have yielded disappointing clinical results. Nevertheless, chemogenetics (gene therapy whereby proteins are engineered to interact with small chemical actuators) has the potential to overcome the limitations of pharmacological interventions. Notably, one such form of chemogenetics is an engineered glutamate-gated chloride (GluCl) channel. (10)
Activation of the GluCl receptor results in chloride influx, leading to neuronal hyperpolarisation and overall suppression of neuronal firing. Importantly, recent data has shown that an enhanced version of GluCl (eGluCl) can decrease seizure activity in rodent epilepsy models. (11) Therefore, given the central role of glutamate in BTRE pathogenesis, there is a unique opportunity to avail of this biochemical autoregulatory gene therapy approach to improve treatment options for brain tumour-related epilepsy.
Research towards the future of BTRE
The Cunningham Laboratory research group aims to understand the basis of neurological and psychiatric disease at the level of the neuronal microcircuit. In particular, our research seeks to understand physiological and pathological electrical activity generated in various disease states. In the BTRE context, our research aims to employ the previously described eGluCl chemogenetic approach to prevent seizure generation and propagation in brain tumour-related epilepsy.
Here, we use closed-loop chemogenetic approaches and wireless electrocorticogram (ECoG) recording methods in a syngeneic orthotopic glioma model. Complementary in vitro brain slice electrophysiological methods (extracellular local field potential (LFP) and whole-cell patch clamp recordings) are employed to study eGluCl treatment effects on harvested peritumoral tissue.
Finally, ex vivo organotypic human peritumoral slices will be generated and used (inter-ictal and ictal activity analysed following treatment) to develop eGluCl to clinical translation. We anticipate this technology will address a significant unmet clinical need, providing effective therapeutic options in drug refractory BTRE patients.
Acknowledgements
This work has emanated from research supported in part by research grants from the Medical Research Council UK (MR/X004317/1) and Science Foundation Ireland (SFI) under Grant Number 20/FFP-P/8613 (Frontier for the Future project award) and Grant Number 16/RC/3948 and co- funded under the European Regional Development Fund and by FutureNeuro industry partners.
References
- Dührsen, L. et al. Seizures as presenting symptom in patients with glioblastoma. Epilepsia 60, 149–154 (2019).
- Sherman, J. H. et al. Impact of temozolomide chemotherapy on seizure frequency in patients with low- grade gliomas: Clinical article. J Neurosurg 114, 1617–1621 (2011).
- Maschio, M. Brain Tumor-Related Epilepsy. Current Neuropharmacology vol. 10 (2012).
- Klein, M. et al. Epilepsy in Low-Grade Gliomas: The Impact on Cognitive Function and Quality of Life. Ann Neurol vol. 54 (2003).
- Pallud, J. et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med 6, (2014).
- Barker-Haliski, M. & Steve White, H. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med 5, 1–15 (2015).
- Tabatabaee, M. & Menard, F. Glutamate Signaling and
Filopodiagenesis of Astrocytoma Cells in Brain Cancers: Survey and Questions. Cells vol. 11 Preprint at https://doi.org/10.3390/cells11172657 (2022). - Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019).
- Smeaton, M. Epilepsy, brain tumours and pioneering neurosurgery. Open Access Government (2022).
- Frazier, S. J., Cohen, B. N. & Lester, H. A. An engineered glutamate-gated chloride (GLUCL) channel for sensitive, consistent neuronal silencing by ivermectin. Journal of Biological Chemistry 288, 21029–21042 (2013).
- Lieb, A. et al. Biochemical autoregulatory gene therapy for focal epilepsy. Nat Med 24, 1324–1329 (2018).

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.