New clinical trials in brain health require innovative methodologies for targeted recruitment and longitudinal assessment. Professor Anne Corbett outlines how her team’s PROTECT portfolio can overcome challenges in trials of cognitive health interventions, offering solutions for intelligent trial design
After decades of relative inactivity, there is currently a growing interest and investment in novel interventions for cognitive and mental health conditions. This is particularly true for trials in cognitive impairment and early dementia. This health area has previously been considered too high-risk and challenging, with failed trials and difficulty in recruiting target groups leading to widespread demotivation for trials in this area. However, a new generation of disease-modifying treatments for Alzheimer’s Disease is in the pipeline, with immunotherapy lecanemab receiving FDA approval in 2023 and donanemab currently under review. (1) These successes have reinvigorated development in Alzheimer’s and dementia drug discovery programmes, and larger numbers of trials are anticipated in the coming years.
A major challenge in trials of cognitive health interventions is in the objective measurement of cognitive performance, both for screening purposes and for measurement of progression through a trial to detect treatment response. The new wave of treatment trials will require the recruitment of people with pre-clinical or very early cognitive decline rather than established dementia. Yet traditional assessment tools such as the ADAS-Cog, which are commonly used in Alzheimer’s trials, lack the sensitivity to detect these states, and other diagnostic tools such as biological biomarkers and neuroimaging are too costly and time-consuming to use at scale in large phase III studies. There is also a drive for less clinically demanding assessment protocols that can be conducted outside of specialist assessment settings.
FLAME cognitive test
Computerised cognitive assessment offers a valuable solution for these trials of the near future. Systems such as our FLAME test battery are built on decades of research and validation and provide objective, sensitive, accurate measurement of cognition. FLAME can be completed unsupervised at home, enabling multiple timepoint assessments in large numbers of participants and providing an unprecedented level of granularity in cognitive data for trial outcomes. With cognitive domains spanning memory, attention, and executive function, FLAME offers a means of exploring multiple cognitive variables in addition to global cognitive endpoints. This is likely to prove valuable in trials for different dementia types, for example, Parkinson’s Disease dementia or Frontotemporal dementia, where non-amnestic impairments are more prevalent.
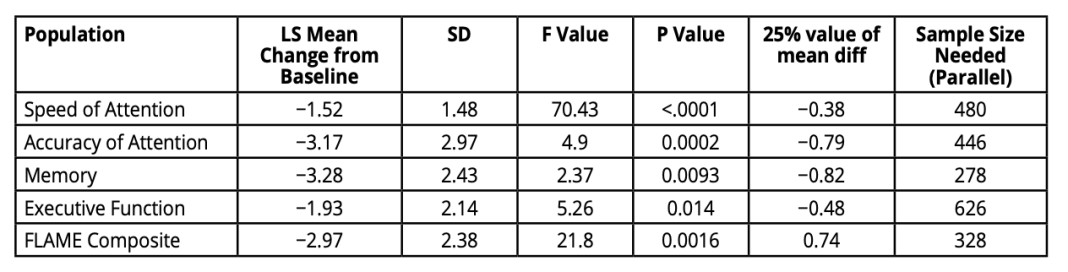
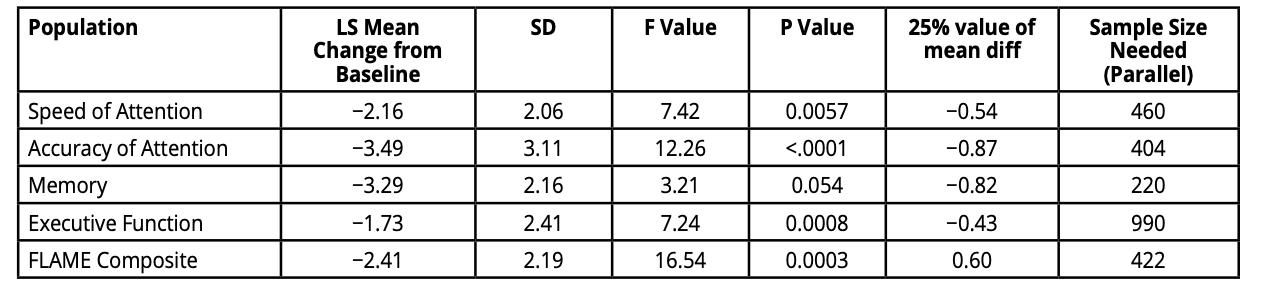
The sensitivity of computerised cognitive test systems such as FLAME also enables the refinement of sample size calculations for trials since they offer more powerful outcome measurements. We have explored the sample sizes required to provide 80% power to a 0.05 level of significance to detect a 25% treatment effect in trials in people with either amnestic or executive MCI. Previous estimations using the ADNI dataset have reported requiring over 1000 participants per arm to achieve this. (2) Using composite outcome measures collated with the FLAME battery, sample sizes can be reduced to less than 200 per arm, thus making trials more affordable and better powered than with less sensitive alternative measures. (Table 1 & 2). (3)
FLAME is already in use as an exploratory outcome measure in two commercial trials of early dementia treatments and as a primary outcome measure in three further trials in early Alzheimer’s Disease and post-operative cognitive decline. Earlier trials delivered by our team have successfully delivered very large-scale online intervention studies using computerised cognitive assessment, which illustrate the power of this approach to trials. For example, our trial of cognitive training in older adults recruited and assessed 6742 participants, including 2873 with pre-clinical cognitive impairment in a study that was delivered entirely remotely. (4) Furthermore, screening of computerised cognitive data from participants in our PROTECT-UK ageing cohort has enabled us to recruit over 60,000 participants for clinical trials in the UK. The remote cognitive testing protocol also has considerable value in the context of the recent COVID-19 pandemic. Where other trials were forced to halt recruitment and assessment, our team recruited and completed a major trial of a nutritional supplement in 620 people through online and digital means. (5) Participant feedback for this trial was enormously positive, with people reporting a preference for the remote, at-home assessment approach.
New generation of clinical trials in brain health
A new generation of trials in brain health is rapidly emerging. Innovative methodologies are required to enable targeted recruitment and sensitive longitudinal assessment of trial cohorts. Remote computerised assessment of brain health offers the opportunity for high-quality data, increased statistical power, and improved participant experience. Our PROTECT portfolio provides solutions for intelligent, innovative trial design and computerised assessment, with a growing collaborative network spanning Europe and the US.
References
- https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval
- https://pubmed.ncbi.nlm.nih.gov/22503160/
- https://pubmed.ncbi.nlm.nih.gov/33088895/
- https://pubmed.ncbi.nlm.nih.gov/26543007/
- https://www.isrctn.com/ISRCTN79265514?q=&filters=%20conditionCategory:Mental%20and%20Behavioural%%2020Disorders,trialStatus:Ongoing&sort=&offset=7&%20totalResults=293&page=1&pageSize=10&searchType%20=basic-search

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.