Gratian Ting and Arpita Bose from Washington University in St. Louis discuss the fascinating role that extracellular electron transfer plays within the human gut
The human gut microbiome contains around hundreds of trillions of bacteria, and a great diversity of species. In fact, the number of bacteria in the human gut is similar in number to all the cells in the human body, further signifying the heterogeneity and significance of this microbiota. The habitat filtering of the human gut, defined as the non-random survival of microorganisms in relation to characteristics of the surrounding environment, is influenced by two important factors: host and diet. Disruption of normal host gut processes through various means, could cause cell death and dysbiosis, disrupting host-mediated habitat filtering. However, diet plays a far more common role in this process. Different dietary behaviors exist between individuals from cultural, moral, economic, and other means.
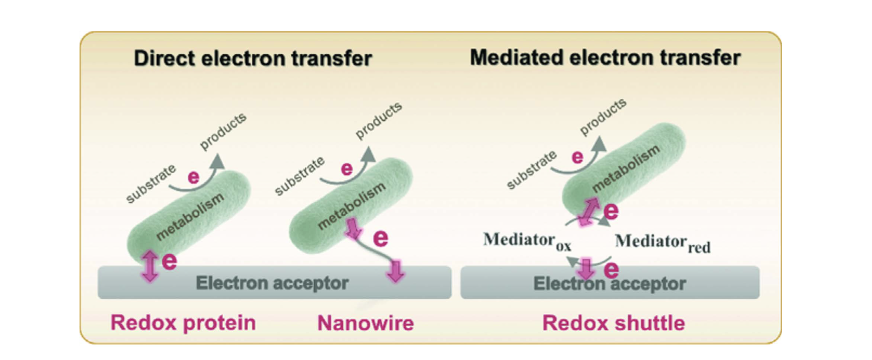
Gut microorganisms metabolize organic compounds for energy, and an important part of this process requires the transfer of electrons to electron acceptors. To get into specifics, the small intestine – a part of the gut – is mostly composed of facultatively anaerobic bacteria. By providing oxygen as an electron acceptor into the gut through human respiration and circulation, the host can oxidize diet derived sugars, amino acids, fatty acids, etc. in the small intestine. In recent years, some gut bacteria have been reported to be electrochemically active bacteria (EAB) as they are capable of utilizing extracellular electron transport (EET) (Figure 1).
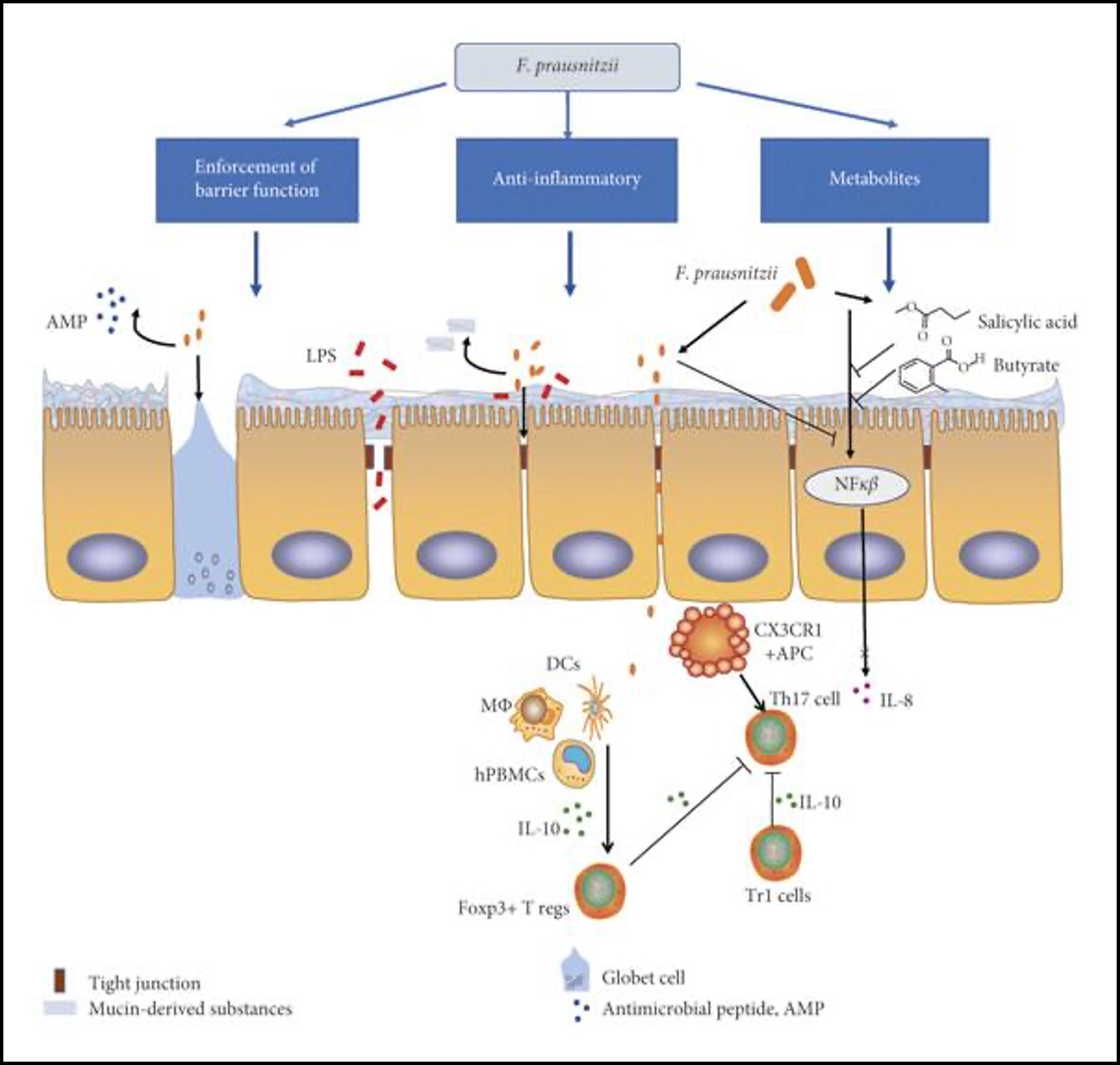
Faecalibacterium prausnitzii, is a bacterium known for its anti- inflammatory capabilities and for being one of the most abundant commensal bacteria in the human colon. Lower F. prausnitzii numbers are found in inflammatory bowel disease illustrating its importance in human gut health. F. prausnitzii is one of the key butyrate producers found in the intestine. Butyrate plays an important role as it can inhibit activation of certain transcription factors (e.g. NF-κB or cytokine interferon gamma) and ultimately reduce intestinal mucosa inflammation making it an important metabolite in this environment for the host (Figure 2). Though F. prausnitzii is a strict anaerobe and consequently extremely oxygen sensitive, it is able to tolerate low concentrations of oxygen found in the intestinal mucosa by using extracellular electron transfer. F. prausnitzii uses EET via flavins and thiols to oxygen to grow at oxic-anoxic interfaces. Being an anaerobe, EET to oxygen might allow
F. prausnitzii to mitigate oxidative stress as it would create a more anoxic environment. This might explain F. prausnitzii’s ability to adhere to oxygen rich epithelial cells in the gut mucosa despite being an anaerobe. The human gut has an abundance of flavins and thiols as well as oxygen that diffuses from epithelial cells.
These relationships that apply to natural gut microorganisms might also apply to the pharmaceutical field, as probiotic bacteria in over-the-counter supplements have the capability to perform EET. The strains Lactobacillus plantarum WCFS1 and Lacticaseibacillus rhamnosus GG are noted for their probiotic effects by decreasing proinflammatory cytokine expression and improving epithelial barrier function. More interestingly L. plantarum can use EET to generate electrical currents and reduce iron(III). EET results in greater ATP production and higher NAD+/NADH ratio. EET has also been found to improve the adherence of probiotic bacteria to intestinal cells. EET in L. plantarum, could enhance the colonization effectiveness of Caco-2 cells (Colorectal Adenocarcinoma Cells).
Bacterial EET activity in the intestine doesn’t always bring positive impacts.
For example, Listeria monocytogenes (a pathogen that is found in contaminated foods) utilizes a flavin based system for EET (FLEET). The deficiency of ndh2 – which encodes an NADH dehydrogenase – impairs L. monocytogenes colonization of the murine digestive tract. Enterococcus faecalis also uses FLEET genes for metabolism and for generating electrical currents, primarily using iron as an electron acceptor. Pathogens, like Enterococci or Listeria, might use EET for fitness, making infectious cases often chronic after the development of a biofilm. The increased biofilm matrix depths because of EET could bring further resistance to medicinal treatments like antibiotics. Overall, extracellular electron transfer plays an important role in the human gut, whether harmful or beneficial. Consequently, its interesting capabilities, especially in fields of medicines, should call for further research.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.