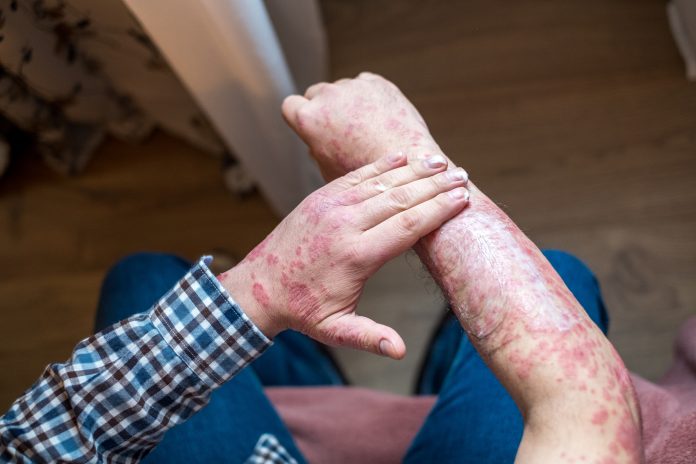
Inflammatory skin diseases like psoriasis involve interactions between skin cells (keratinocytes) and immune cells, which leads to chronic inflammation, disfiguring skin lesions, and systemic effects
The usual treatment for this relies on immunosuppressants; this tends to cost a lot and can have significant side effects.
A recent study by Winge et al. looks at treating psoriasis without relying on immune suppression.
The researchers determined benzamil, as a potential candidate for limiting inflammatory skin disease.
Treating psoriasis: A chronic inflammatory skin disease
Psoriasis is a common autoimmune condition characterised by the rapid turnover of skin cells, leading to thick, scaly plaques on the skin.
This is mostly due to dysregulation in immune responses, where T cells and other immune cells release pro-inflammatory cytokines, prompting keratinocytes to proliferate abnormally.
This inflammation is further worsened by signaling pathways, such as the ones negotiated by the transcription factors NF-κB and STAT3.
Psoriasis affects the skin and can also be associated with systemic conditions, including cardiovascular disease, making it important to find a treatment to improve overall health.
Current treatments for psoriasis include topical corticosteroids, biologics targeting specific immune pathways, and systemic immunosuppressants.
However, these treatments often have limitations, including side effects (e.g., immune suppression, increased infection risk) and high costs.
This makes researchers keen to find new treatments that can directly target inflammatory processes in keratinocytes.
Using screening to find new drug targets
The study by Winge et al. used a computational approach to screen existing FDA-approved drugs to identify what is capable of reversing the inflammatory gene expression signature in keratinocytes.
By analysing transcriptomic data, they focused on compounds that could modulate the inflammatory pathways typically activated in psoriasis. The screening led to identifying benzamil, which had not been previously linked to treating inflammatory skin diseases.
Mechanism of action of benzamil
Benzamil was found to work by disrupting key signaling mechanisms in keratinocytes, especially through its effects on intracellular pH. By lowering intracellular pH, benzamil interfered with activating the small GTPase Rac1, a protein crucial for many inflammatory processes.
Rac1 normally interacts with the adaptor protein NCK1, which enables downstream inflammatory signaling through pathways like STAT3 and NF-κB. Benzamil hindered the binding of Rac1 to NCK1, reducing activation of these pro-inflammatory pathways and altering cytokine production in keratinocytes.
The authors confirmed the efficacy of benzamil in three independent mouse models of skin inflammation: Rac1G12V transgenic mice, a mouse model treated with topical imiquimod (a compound that induces psoriasis-like inflammation), and human skin xenografts derived from patients with psoriasis.
In all these models, systemic and topical treatments with benzamil effectively reduced inflammation and keratinocyte hyperproliferation, suggesting that benzamil could be a possible treatment option for psoriasis.
Therapeutic potential of benzamil
A key takeaway from this study is that benzamil does not involve systemic immune suppression.
Unlike traditional treatments for psoriasis, which can leave patients vulnerable to infections and other side effects, benzamil acts directly on the keratinocytes, the primary cells involved in the inflammatory process. By targeting the Rac1-NCK1 interaction, benzamil could limit inflammatory signaling without affecting the broader immune system.
The study further demonstrated that both systemic and topical applications of benzamil were effective. This approach makes it a universal therapeutic option, as it could be tailored to individual patients based on the severity and location of their psoriasis.
The study by Winge et al. opens the door to new treatment for psoriasis, which would focus on keratinocyte-driven inflammation rather than immune suppression.
Future clinical trials will be needed to confirm its safety and efficacy in human patients, but these early results are promising for patients looking for alternatives to current immunosuppressive therapies.