Priya Hays, PhD, CEO of Hays Documentation Specialists, discusses innovations and advancements in the development and evaluation of personalized cancer therapies
Advancements in cancer research have led to changes in the treatment of patients with cancer, resulting in novel personalized cancer therapies. The three major classes include small molecule inhibitors, biologics, and cancer immunotherapies. Standard first-line immunotherapies include anti-CTLA-4 inhibitor ipilimumab and anti-PD-1 inhibitor nivolumab. These are considered immune checkpoint inhibitors that remove the breaks in cytotoxic T cells from killing cancer cells. Due to the incidence of non-responders to these first-line immunotherapies, other immunotherapies also belonging to the same immune checkpoint inhibitor class of treatments have been developed and evaluated in clinical trials, and novel therapeutic agents have been developed that could serve as the next-generation immune checkpoint inhibitors, which include anti-LAG-3 and anti-TIGIT antibodies and the inhibitory receptor TIM-3. LAG-3 has been named ‘the third checkpoint inhibitor.’ Clinical trials have been conducted to study and evaluate these three agents. ‘These inhibitory receptors have heralded the ‘next wave of co-inhibitory receptor targets that are being explored in clinical trials,’ as stated in prior reports. (1)
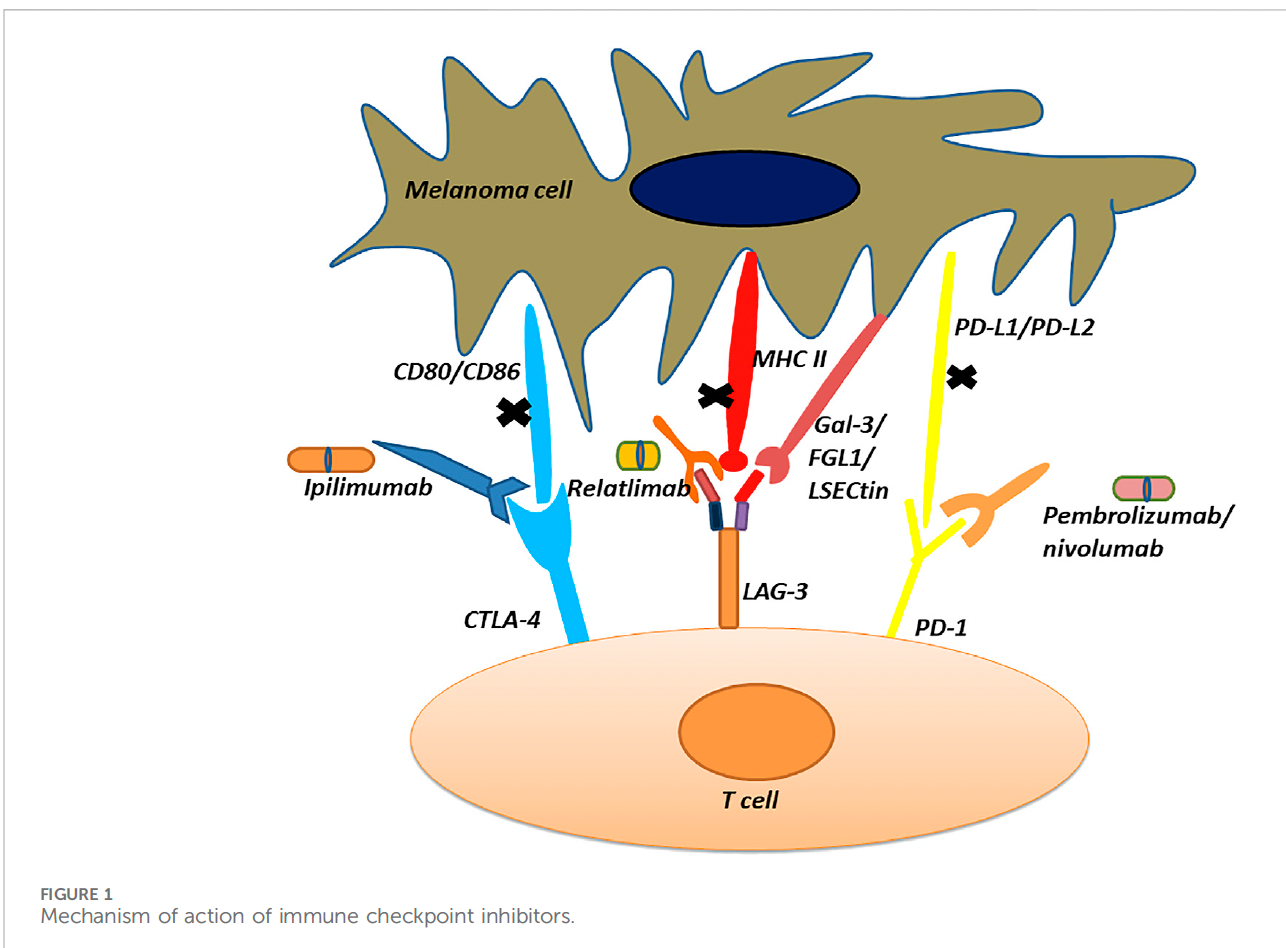
In vitro testing in activated T cells led to the discovery of LAG-3 as an inhibitory receptor, similar to PD-1/PD-L1 and CTLA-4. Like PD-1/PD-L1, LAG-3 inhibits T cell function, thereby its cytotoxic activity and inhibiting CD4 T cell activation. The phase III RELATIVITY-047 trial was recently conducted and led to the FDA approval of the LAG-3 antibody relatlimab for advanced melanoma patients.
One report noted: “Following the approval of relatllimab, the FDA approved an agent that combines anti-LAG-3 mAB relatlimib with nivolumab for first-line treatment of metastatic melanoma in a phase 2–3, global, double-blind, randomized trial evaluating fixed-dose combination as compared with nivolumab alone when administered intravenously every four weeks to patients with previously untreated metastatic or unresectable melanoma. The primary endpoint was progression-free survival.’’ (2)
Other inhibitory receptors are the TIGIT antibody and the Tim-3 antibody. The TIGIT inhibitory receptor and PD-1 are ‘mechanistically convergent checkpoints that regulate T cell differentiation,’ and blockade through anti-TIGIT and PD-1 antibodies leads to the reversal of a tumor microenvironment phenomena known as T cell exhaustion and expansion of the cytotoxic T cell compartment.
T cell immunoglobulin-3, or Tim-3, is expressed on interferon gamma-producing CD4+ helper and CD8+ cytotoxic T cells and serves as a cell surface marker. One monoclonal anti-TIM-3 antibody has been developed, showing clinical activity in non-small cell lung cancer and melanoma patients through a unique mechanism of action by blocking the TIM-3 interaction with a phosphatidylserine ligand. This agent, known as Sabatolimab, has been evaluated with an anti-PD-1 antibody known as spartaliuzmab in a phase 1 trial for determining safety profiles and tolerability. (2)
Breast cancer case study
Case Study: PARP inhibitors (poly (ADP-ribose) polymerase inhibitors) and CDK4/6 inhibitors (cyclin-dependent kinase 4 and 6 inhibitors) that treat advanced and metastatic breast cancer.
PARP inhibitors olaparib, talazoparib, and veliparib and CDK4/6 inhibitors abemaciclib, palbociclib, ribociclib, are considered first-line standard of care for the treatment of advanced breast cancer, each agent has different mechanisms of action and clinical efficacies. PARP inhibitors inhibit the enzyme PARP1 that repairs DNA double stranded breaks preventing cancer cell growth. CDK4/6 inhibitors impact the cell cycle and also prevent cancer cell growth and are usually administered with aromatase inhbitors. (3,4)
Conceptually, the management of advanced breast cancer patients could be potentially complex and involve a lengthy decision-making process choosing between these agents. However, most of the literature on this topic is sparse. Several recent clinical trials have evaluated the various agents in both classes, and the data revealed robust clinical outcomes and manageable safety profiles for both.
The BROCADE trial showed that the PARP inhibitor veliparib in combination with chemotherapy was effective for locally advanced breast cancer patients with germline (inherited) BRCA1/2 mutations (gBRCA1/2) with increased median progression free survival, a statistical indicator of clinical success, by nearly three months (7.0 months vs. 4.2 months). The EMBRACA trial likewise showed clinical effectivity for tumors harboring the gBRCA1/2 mutation for talazoparib, another PARP inhibitor, compared to standard of care (8.6 months vs. 5.6 months). The OlympiAD trial showed clinical effectiveness for olaparib.
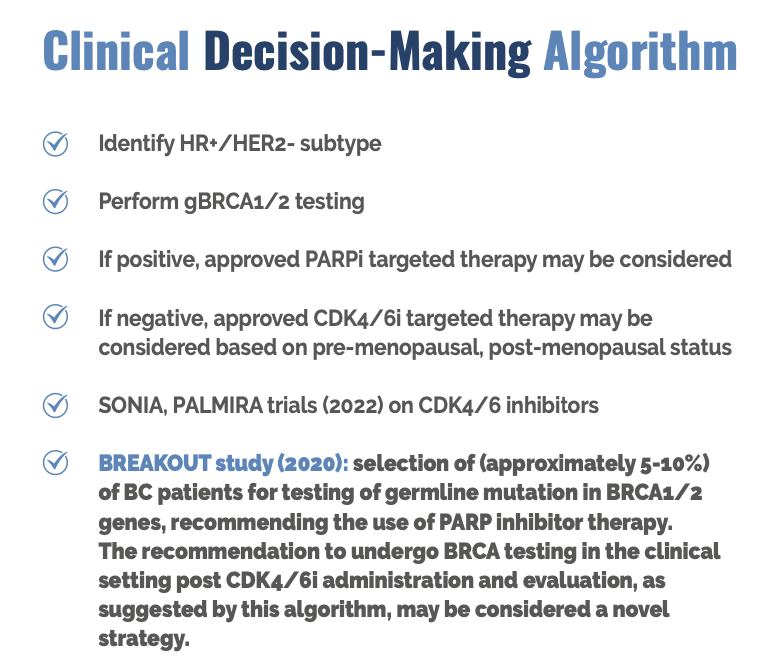
The MONARCH trial showed that abemaciclib, a CDK4/6 inhibitor, had a significantly longer duration of progression-free survival when compared to the placebo (median not reached in the abemaciclib arm, 14.7 months in the placebo arm.) The MONALEESA trial showed that ribociclib, also a CDK4/6 inhibitor, had significantly longer mPFS when compared to placebo. Adverse clinical outcomes for CDK4/6 inhibitors were shown in two phase III trials, the SONIA and PALMIRA, suggesting the use of PARP inhibitors for breast cancer patients after testing positive for the gBRCA1/2 mutation.
The BREAKOUT study conducted in 2020 had an intriguing result that implied that advanced breast cancer patients undergo gBRCA1/2 testing after receiving CDK4/6 inhibitors when they are not effective in this population.
This stemmed from data that showed that 5-10% of advanced breast cancer patients were positive for the gBRCA1/2 mutation in the clinical setting with the workflow shown below. (6)
Much of this innovation and progress in developing and evaluating personalized cancer medicines demonstrate the unique nature of precision oncology: personalized cancer care is based on the instances of non-responders in immune checkpoint inhibitors. Small molecule inhibitor drug development results from mutations in oncogenes that lead to drug resistance. Each healthcare system, including the NHS, has mechanisms for developing and adopting these therapies, and each plays a distinct role in implementing them for patient care.
Please seek permission from the author before using this content
References
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT coinhibitory receptors with specialized functions in immune regulation. Immunity. 2016; 44: 989-1004.
- Hays P. Immune checkpoint blockade response and resistance: Next generation therapeutic agents for solid tumors, Front Immunol Immuother. 2023; 2: 107.
- Abdelmalak M, Singh R, Anwer M, Ivanchenko P, Randhawa A et al. The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance. Cancers 2022, 14, 5388.3.
- Menezes MCS, Raheem F, Mina L, Ernst B, Batalini F. PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond. Cancers 2022, 14, 4332.
- Banta Karl L. Banta, Xiaozheng Xu, Avantika S. Chitre, …, Enfu Hui, Eugene Y. Chiang, Ira Mellman et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022. 55. 512-526.
- Nadine M. Tung and Judy E. Garber. BRCA1/2 testing: therapeutic implications for breast cancer management. British Journal of Cancer, 2020.
- Priya Hays. (2024) “Clinical Decision Making in Advanced and Metastatic Breast Cancer Cases between PARP Inhibitors and CDK4/6 Inhibitors: A Review.” Clinical Case Reports and Clinical Study, 11(2).
- SuJ,FuY,CuiZ,AbidinZ,YuanJ,ZhangX,LiRand Zhao C (2024), Relatlimab: a novel drug targeting immune checkpoint LAG-3 in melanoma therapy. Front. Pharmacol. 14:1349081. Lung Cancer


