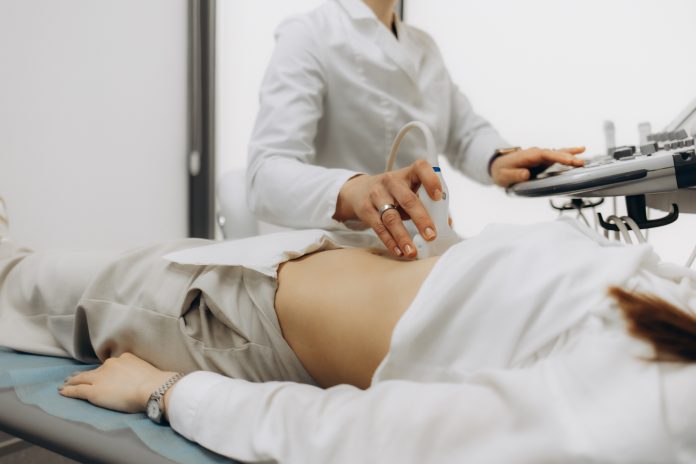
University Hospitals Coventry and Warwickshire (UHCW) NHS Trust is to lead a clinical trial of a novel device aimed at addressing miscarriage symptoms
Threatened miscarriage, an unofficial term used by health professionals, is when an expectant mother experiences miscarriage symptoms such as bleeding or pain in the first trimester. Currently, progesterone is given to women experiencing threatened miscarriage.
Experts from UHCW NHS Trust are leading a clinical trial of Callavid®, a device designed to deliver vital doses of progesterone, which could help more than 150,000 women annually in the UK.
World’s first drug-device combination to treat miscarriage symptoms
Callavid® was developed by scientists and biomedical engineers at Calla Lily Clinical Care. They have secured £1 million in Invention for Innovation (i4i) funding from the National Institute for Health and Care Research (NIHR) for its first clinical trial later this year.
The device has a leak-free design, allowing for cleaner and more comfortable progesterone delivery whilst reducing drug wastage.
If approved, the product could become the world’s first drug-device combination product to treat threatened miscarriage.
The trial will be led by consultant Professor Siobhan Quenby MBE – one of the world’s leading authorities on miscarriage and preterm birth – and the team in the Trial Management Unit at UHCW NHS Trust.
Professor Quenby, who specialises in obstetrics and reproductive health at UHCW NHS Trust, said: “New methods to reduce additional psychological anxiety are badly needed in this field. Through this innovation, one which is being pioneered right here in the UK, I believe there is potential to transform women’s experience.”
Callavid® improves the delivery of progesterone medication
Experiencing vaginal bleeding in the early stages of pregnancy is common and does not always mean there is a problem. However, bleeding can sometimes be a warning sign of a miscarriage. A threatened miscarriage is diagnosed by an ultrasound scan, which will detect the baby’s heartbeat. If there is a heartbeat, it is a threatened miscarriage; if there is no heartbeat, then this will confirm a miscarriage.
In many cases, there is no known cause for the bleeding. Still, likely causes may be the placenta trying to burrow itself into the lining of the womb, damage to the cervix, vaginal infection or a small blood clot around the amniotic sac.
NICE recommends the administration of vaginal micronised progesterone for women who have suffered at least one miscarriage previously and face an increased risk of experiencing miscarriage symptoms. It is believed that over 150,000 women in the UK could be eligible for progesterone prescriptions for threatened miscarriage each year.
Progesterone is currently self-administered via vaginal pessaries; however, this method leads to leakage, which can cause significant anxiety for patients during an already stressful time.
Health economists estimate that the avoidable cost to the economy and the NHS from the use of leaky pessaries for miscarriage prevention and IVF in England and Wales is £236m per year.
Dr Lara Zibners, Co-founder and Chair of Calla Lily Clinical Care, said: “The NIHR funding will enable us to test our technology via a full feasibility study this autumn, bringing us one step closer to making this product available to help women at one of the most distressing moments of their lives.”
If approved, Callavid® would become the first drug-device combination product in the UK to be approved by the Medicines and Healthcare Products Regulatory Agency (MHRA) for the treatment of threatened miscarriage.