Professor Colin Suckling of Strathclyde University discusses advancements with Heterocyclic Chemistry and the progress towards new medicines.
Earlier in January I attended a conference at the Roslin Institute near Edinburgh, famous for Dolly the Sheep. Unlike that high profile product of scientific invention and technological virtuosity the subject was on the face of it much more mundane and certainly more down to earth, namely Animal African Trypanosomiasis (AAT). This is the cattle equivalent of sleeping sickness and it is economically devastating in sub-Saharan and savannah Africa because of the toll it takes on herds of cattle which form a principal component of the livelihood of many African farmers. To the credit of the organizers, scientists from around the world came primarily to discuss the biology of the disease which is caused by parasites known as trypanosomes. But my own interest was in a small group of papers at the start of the conference that dealt with the challenges of obtaining new drugs to treat the disease. Drugs are important in this area because it is impossible completely to control the tsetse fly that carries the parasite and because vaccines with wide efficacy are very difficult to obtain for trypanosomes. Moreover such drugs as are available are increasingly falling victim to the development of resistance in the trypanosmes, a typical example of the world-wide problem of antimicrobial resistance.
As readers of any of my previous Special Reports or Profiles will know, my direct interest in this field is exploiting the properties of our specially synthesized heterocyclic compounds as the basis of new medicines to treat AAT and to provide prophylaxis. If AAT can be controlled and cured the economic impact in rural Africa will be huge, certainly improving the lives of many more people than any of the new expensive anti-cancer drugs to say nothing of the benefits to the animals’ health.
Heterocyclic chemistry: the heart of the discovery
Be it human cancer or AAT, heterocyclic chemistry is at the heart of the discovery of new medicines. Our compounds synthesized at the University of Strathclyde and being developed for AAT belong to a class of compound known as minor groove binders (S-MGBs) that engage DNA to kill the trypanosomes in ways yet to be worked out in detail. Our best compounds are very potent but, somewhat surprisingly, are not toxic to mammalian cells and more surprisingly still are effective in mouse models of AAT without evidently causing adverse effects in the mouse. This selective toxicity is what is needed in an anti-infective drug: kill the disease-causing agent but not the host. Moreover, because of the way S-MGBs work it has proved impossible so far to generate resistance to our S-MGBs in laboratory experiments, a very important advantage for a drug that would be widely used in the field over many years.
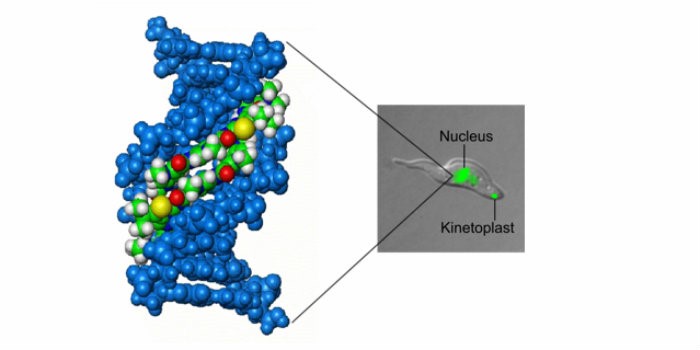
That we can achieve these things is down to the flexibility of heterocyclic chemistry whereby we can make small, subtle changes to the structures of our S-MGBs whilst maintaining the essential properties of binding to DNA and killing trypanosomes. It’s unpredictable what a small change in structure will do. For example, we have found that the addition of just one carbon atom as a methyl group improves the potency of an S-MGB by as much as ten fold. With other compounds, suitable choices of structure provide fluorescence that allow us to follow where the S-MGB goes in the cell and thereby get some information about its mechanism of action (Figure). You don’t find this flexibility of structure and tunability of properties to the same extent in any other field of chemistry.
Hopefully all of these things will come together over the next year or so to provide an S-MGB that can be tested in the real and challenging environment of an African farm and then be developed into a product. To see a new veterinary medicine emerge from an academic laboratory in cold Northern Europe to treat cattle in the heat of Africa would be a powerful demonstration of the ‘long arm of heterocyclic chemistry’.
To find out more, see our project website http://www.gla.ac.uk/researchinstitutes/iii/research/researchareas/parasitology/aat/#/

The anti-AAT project using S-MGBs is a collaborative project between the University of Strathclyde, the University of Glasgow and the Roslin Institute (University of Edinburgh) with major funding from the Biotechnology and Bioscience Research Council. Some key data were obtained at the Swiss Tropical and Public Health Institute (Basel). Advice and a financial contribution have come from the Global Alliance for Livestock and Veterinary Medicine (GALVmed).








