The UK’s medical authorities cleared continued use of AstraZeneca, but say that 18-29 year olds should take an alternative vaccine to AstraZeneca if possible
Last month, the European Medical Agency cleared the vaccine for continued use – after being unable to find a causal link between the very rare incidents of clotting and AstraZeneca.
Today (7 April), UK medical authorities further cleared the vaccine for continued use across the population.
But the guidelines for who can use the vaccine have now changed, to reflect an ongoing and cautious investigation into the connection between blood clot risks and AstraZeneca.
Should under-thirties continue to take AstraZeneca?
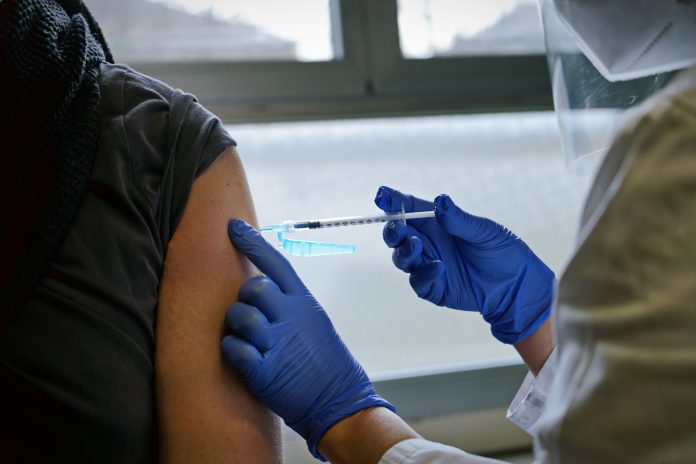
Yes, if you already had the first dose. If you didn’t take anything yet, ask your provider about an alternative.
Instead of giving AstraZeneca doses to the entire population, the Medicines and Healthcare products Regulatory Agency (MHRA) advise generally healthy individuals between the ages of 18 and 29 to opt for an alternative vaccine – if possible.
Those who have already taken one dose of the AstraZeneca vaccine are advised to take their second dose.
The blood clot incidents have impacted 4 in 1,00,000 people, while COVID itself continues to create blood clot related health issues for 23% of patients.
There is continued comparison between the confirmed increased blood clot risk via hormonal contraceptives and the currently unproven rate of incidence in the COVID vaccine.
On 23 March, data from an American trial of over 30,000 people did not find any correlation between blood clots and the vaccine. This trial further confirmed that the vaccine is 100% effective against death via COVID-19.
Professor Van-Tam further explained why 40 to 49 year olds are continuing with AstraZeneca – the risk of ICU admissions would go up by 51.5 if they suspended use of the vaccine.
He described any suspension in this age group as “absurd.” He explained that the blood clot was a “vanishingly rare” side effect, that can only become apparent when “tens of millions” have been vaccinated.
He further suggested that more rare side effects could come to light for every vaccine that is widely used across a population.
Will this change in AstraZeneca use slow UK vaccination?
No, the UK expects enough alternative vaccine deliveries to cover this.
Today, Moderna became available in Wales. The UK at large is expected to receive the Moderna shipment of five million doses and begin use en masse by mid-April.
The use of the Johnson and Johnson vaccine is expected to begin in Summer, with no concrete date yet. This vaccine is a one-dose, meaning that any shipment will go further.
Currently, the UK expected to decrease use of AstraZeneca due to a lack of availability – so the “course correction” described by Van-Tam will not change the expected timeline for full UK vaccination.









What about HRT? Does that effect the likelihood of blood clots?