H. Robert Guy, CEO from Amyloid Research Consultants, ascertains why amyloid oligomer & membrane channels structures can & must be determined
Amyloids are killing us, both literally and financially. Consider just these three: Amyloid β (Aβ) in Alzheimer’s Disease (AD), Α Synuclein (α-Syn) in Parkinson’s Disease (PD) and Amylin (aka IAPP) in Type 2 Diabetes (T2D). The annual worldwide cost of treating these devastating diseases is about a trillion dollars and increasing steadily.
So why are treatments still so inadequate, especially for AD? Part of the problem is that we know so little about the three-dimensional structures of both the functional and toxic forms of amyloid assemblies or even how they affect biological processes, and what many think they know is probably wrong.
Structure-based drug design requires detailed three-dimensional structures of drug-binding sites. When asked about Aβ or α-Syn structures, most researchers think of fibrils associated with plaques or Lewis bodies. But fibrils are not the assemblies that alter the synaptic activity or that decimate mitochondria; oligomers are more toxic. Amyloid oligomers often are characterised as intrinsically disordered. But sequence analyses of distantly related homologs are inconsistent with that perception; they indicate that sometimes at least portions of these oligomers do have highly ordered structures. Sequences of the most hydrophobic portion of Aβ are almost identical in all vertebrates from fish through mammals. Likewise, sequences of the first 62 residues of Α-Syn and β-Syn families are almost the same even though they diverged long ago. Highly conserved hydrophobic portions of homologous proteins usually have similar highly ordered three-dimensional structures.
A more detailed look at my research
I began modeling the structures of membrane channels in the mid- 1970s, beginning with β barrel models of bacterial porin, then moved on to transmitter receptors, voltage-gated K+, Na+, and Ca2+ and inward rectifying channels, K+ transporters, VDAC, mechanosensitive channels (MscL and Trek), antimicrobial peptides (magainin and cecropins), toxic peptides (melittin) and even shark repellent (pardaxin). My group began collaborating with Harvey Pollard and Nelson Arispe in the mid-1990s when they discovered that Aβ forms membrane channels. We published our concentric β barrel models of Aβ oligomers, annular protofibrils and channels in 2010. Recently, we extended this concept to α-Syn and amylin channels and are revising and updating our Aβ models.
Small Aβ oligomers in water appear to be disordered. However, as the number of subunits in the assemblies increases and/or as the environment around the assemblies becomes hydrophobic due to interactions with fatty acids, lipids and membranes; the assemblies likely become more ordered. The increased order is due to increased hydrogen bonding among backbone atoms. Polar backbone atoms can H-bond to water in an aqueous environment, but in a hydrophobic environment, they are more likely to bind to each other to adopt α helical and/or β sheet secondary structures. Backbone H-bonds of α helices form within each monomer (intra-subunit), in β structures they can bind subunit together (inter-subunit). Thus, α helices are likely to form when monomers bind to membranes or the oligomers have few subunits, but β secondary structures are more likely to stabilise large assemblies by binding adjacent subunits together. This appears to occur in amyloids. Amylin, Aβ and Α-Syn all have regions that form α helices in small assemblies and when monomers bind to membranes, but form β-sheets in large assemblies.
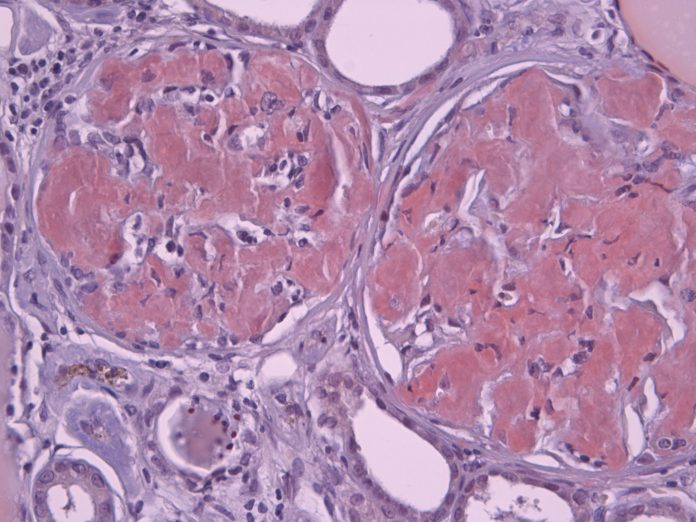
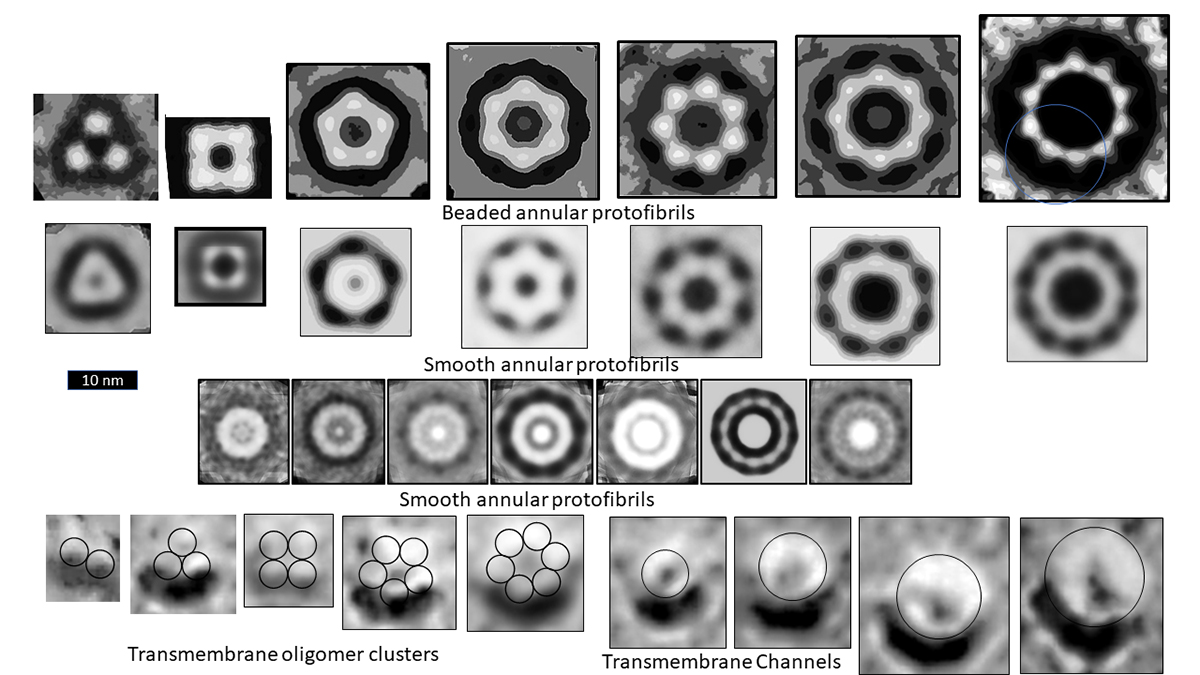
Microscopy images of the oligomer, protofibril and channel structure of Aβ, Α-Syn, and amylin indicate multiple sizes of assemblies. The figure illustrates images of three categories of Aβ Annular Protofibrils (APFs) and two forms of transmembrane assemblies. We propose that each bead of beaded APFs is an oligomer (hexamers in these images). These beaded APFs gradually morph into smooth APFs. Two categories are illustrated, one with a black centre and one with a white centre. Those with black centres are more like the b-APFs from which they develop. Concentric rings are more apparent in the white-centred APFs. Each ring corresponds to a β barrel in our models. Freeze fracture images of transmembrane Aβ assemblies show similar rings of beads and smoother, circular assemblies. We model the beads as transmembrane oligomers with concentric β barrels and postulate that, like APFs, they morph into channels with only one set of concentric barrels.
Our models also suggest that amyloid channels could be mechanosensitive and/or contribute to the formation of fusion pores. These mechanisms could help explain the role amyloids play in transmitter release and endocytosis at synapses and in insulin release in β cells. Mitochondria swelling and fission contributes to cell death in many degenerative neurological diseases and in T2D. Deleterious effects of Α-Syn on mitochondria are well documented: they involve the formation of transient transition pores. Although less studied, Aβ and amylin also concentrate in mitochondria membranes and increase their permeability. Nonetheless, to our knowledge, no one has studied whether conductance through any of these amyloid channels increases when the membranes are stretched or whether they contribute to the formation of fusion pores and membrane fusion.
Looking ahead
We still have much to do to understand the structures of both functional and toxic amyloid assemblies. Our models are detailed hypotheses that can be tested in numerous ways. The micrographs we have used to constrain our models were developed over a decade ago. It should be possible to obtain higher resolution results using contemporary methods, such as cryo-microscopy and single particle analyses. The goal of understanding the precise structures of these assemblies is not hopeless. It’s well past time to support these efforts. The potential benefits in alleviating human suffering and reducing medical costs would be enormous.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.