Genital Chlamydia trachomatis infection is a significant public health burden; Professor of Pediatrics and Microbiology and Immunology, Toni Darville, discusses the potential efficacy of CPAF-adjuvanted vaccines in mitigating the spread and impact of the infection
Chlamydia trachomatis (CT) is the most common sexually transmitted bacterial infection globally, with over 100 million cases annually and direct treatment costs in the United States exceeding $500mn. (1) In women, CT infects the endocervix but can spread to the uterus and oviducts to cause symptomatic (~10%) or subclinical (~25%) pelvic inflammatory disease (PID). PID affects ~800,000 American women annually, and yearly treatment expenditures approach ~$2bn. (2) Sequelae include chronic pelvic pain (30%), infertility (10%), and ectopic pregnancy (10%). Because most (70%) of CT infections are asymptomatic, a major barrier to preventing CT disease is delayed diagnosis, but both clinical and subclinical upper-tract inflammation can lead to chronic oviduct damage despite the availability of effective antibiotic therapies. (3) Therefore, effective vaccines are urgently needed to control transmission and prevent reproductive tract disease.
CD4 T-cell immunity protects against genital Chlamydia infection
Natural immunity in humans indicates the importance of CD4 T cells for resolution of CT infection and protection from reinfection. (4,5) The Darville laboratory at the University of North Carolina at Chapel Hill (UNC-CH) has conducted longitudinal studies on CT-exposed women enrolled in the T cell Response Against Chlamydia (TRAC) study and has shown that memory interferon-gamma (IFNg)+ CD4 Th1, Th17, and Th1/Th17 cells, are crucial to resistance to reinfection,(6) while anti-CT antibodies do not correlate with protection. (7)
CT is a highly prevalent pathogen, and its success is, in a large part, due to its ability to evade host immune responses. It invades host epithelial cells through multiple mechanisms, thereby prohibiting effective in vivo neutralization. Once inside, it grows and replicates within a protective intracellular vacuole, minimizing exposure to the host immune system. Effective chlamydial killing requires the induction of chlamydial-specific T cells that produce IFNg, which can starve the organism of essential nutrients and activate professional phagocytes for chlamydial killing. Immunological profiling strongly indicates that a protective subunit vaccine will be comprised of highly immunogenic antigen(s), delivered using a platform that induces robust, local, and systemic memory IFNg-producing CD4 T cell responses. By probing the chlamydial proteome for antigens that are broadly and highly recognized by CT-exposed women, the Darville laboratory has determined that Chlamydial Protease Activation Factor (CPAF), a conserved CT virulence factor, is the top immunogenic protein recognized by antibody and CD4 T cells, (7,8) far outcompeting other CT proteins. CPAF is a potent CD4 T cell antigen containing multiple MHC class II epitopes and has high sequence conservation between CT serovars, making it an ideal candidate for a monovalent subunit vaccine. (8)

A chlamydia vaccine? Preclinical murine studies document potential efficacy of CPAF-adjuvanted vaccines
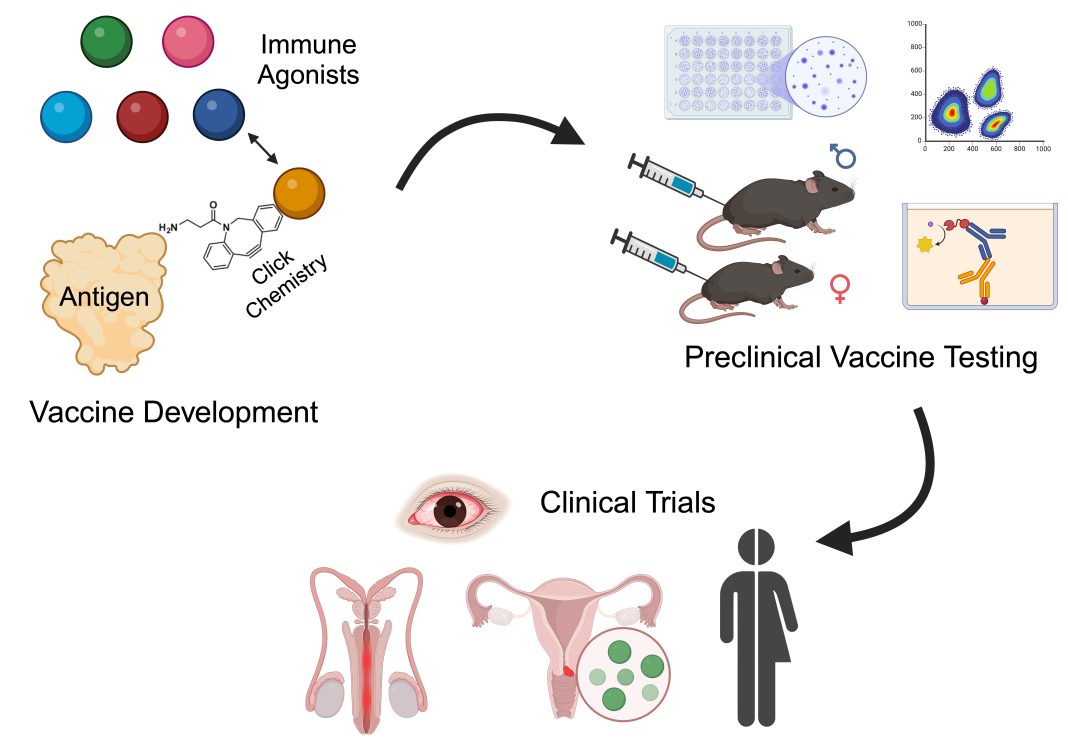
Although attenuated chlamydial vaccines induce robust T cell responses and reduce chlamydial genital infection and disease in animals, (9) inherent safety issues raise concern for their use to prevent human genital infections. To address this shortcoming, Drs Toni Darville and Taylor Poston at UNC-CH developed a collaboration with Drs Jeff Fairman and James Rozelle at Vaxcyte, Inc., and Dr Aaron Esser-Kahn at the University of Chicago to develop a safe and efficacious subunit vaccine for humans. The team has developed an approach to generate subunit vaccines comprising enzymatically inactivated CPAF, directly conjugated to Toll-Like Receptor (TLR) or STimulator of INterferon Genes (STING) agonists, allowing for potent and tunable mucosal immune responses (FIGURE). This novel covalent CPAF-adjuvant conjugation approach promotes antigen-presenting cell activation through coincident stimulation, leading to reduced toxicity, enhanced immunogenicity, and superior efficacy, with the potential for dose-sparing and reduced cost of goods.
Immunization with CPAF admixed with the STING agonist (ADU-S100) was well-tolerated in female mice, induced a CPAF-specific CD4 T cell response, and provided significant protection from infectious burden with a promising trend for reduced oviduct pathology. (10) Recent unpublished data revealed that immunization with CPAF covalently conjugated to a novel STING agonist provided equivalent immunogenicity and protection to admixture using 25-fold less agonist. Challenge studies comparing the conjugate vaccine to admixed formulations via mucosal and systemic routes are ongoing.
Creating a chlamydia vaccine: A path forward to human clinical trials
Vaxcyte’s site-specific in vitro transcription-translation platform enables the conjugation of immune agonists to a vaccine immunogen at specific sites and on a large scale. This system avoids interference with immunodominant epitopes, protects the physical properties of the antigen, and can be scaled up easily while avoiding batch-to-batch variability. A three-way partnership (PHOTO/ Figure) between Drs Darville and Poston (UNC-CH), with expert knowledge of chlamydial immunobiology, antigen discovery, and murine preclinical vaccine testing; the Esser-Kahn group (U Chicago), who has extensive expertise in the design and testing of synthetic small molecule TLR and STING agonist conjugates; and Vaxcyte, Inc., with expertise in vaccine antigen and platform manufacture, and investigational new drug enabling studies and regulatory matters, provides a direct path forward to generate and test additional CPAF- adjuvant conjugates for superior immunogenicity and protection capabilities, minimized toxicity, and ease of production and delivery, with a goal of advancing to human clinical trials.
References
- Owusu-Edusei K, Jr., Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MC, Kent CK: The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013, 40:197-201.
- Rein DB, Kassler WJ, Irwin KL, Rabiee L: Direct medical cost of pelvic inflammatory disease and its sequelae: decreasing, but still substantial. Obstet Gynecol 2000, 95:397-402.
- Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL: Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol 2012, 120:37-43.
- Bakshi RK, Gupta K, Jordan SJ, Chi X, Lensing SY, Press CG, Geisler WM: An Adaptive Chlamydia trachomatis- Specific IFN-gamma-Producing CD4(+) T Cell Response Is Associated With Protection Against Chlamydia Reinfection in Women. Front Immunol 2018, 9:1981.
- Russell AN, Zheng X, O’Connell CM, Wiesenfeld HC, Hillier SL, Taylor BD, Picard MD, Flechtner JB, Zhong W, Frazer LC, Darville T: Identification of Chlamydia trachomatis Antigens Recognized by T Cells From Highly Exposed Women Who Limit or Resist Genital Tract Infection. J Infect Dis 2016, 214:1884-92.
- Yount KS, Kollipara A, Liu C, Zheng X, O’Connell CM, Bagwell B, Wiesenfeld HC, Hillier SL, Darville T: Unique T cell signatures are associated with reduced Chlamydia trachomatis reinfection in a highly exposed cohort. bioRxiv 2023.
- Liu C, Hufnagel K, O’Connell CM, Goonetilleke N, Mokashi N, Waterboer T, Tollison TS, Peng X, Wiesenfeld HC, Hillier SL, Zheng X, Darville T: Reduced Endometrial Ascension and Enhanced Reinfection Associated with IgG Antibodies to Specific Chlamydia trachomatis Proteins in Women at Risk for Chlamydia. The Journal of Infectious Diseases 2021.
- Li Y, Warren JA, Poston TB, Clutton G, Shaw FR, Conrad SZ, Xu Y, Zheng X, Yount KS, O’Connell CM, Wiesenfeld HC, Darville T, Goonetilleke N: Chlamydia trachomatis induces low-frequency, sustained CD4 T cell responses in most women, predominantly targeting chlamydial protease-like activity factor, CPAF. J Infect Dis 2024.
- O’Connell CM, Ingalls RR, Andrews CW, Jr., Scurlock AM, Darville T: Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 2007, 179:4027-34.
- Poston TB, Girardi J, Kim M, Zwarycz P, Polson AG, Yount K, Hanlan C, Salas IJ, Lammert SM, Arroyo D, Bruno T, Wu M, Rozzelle J, Fairman J, Esser-Kahn A, Darville T: Intranasal immunization with CPAF combined with cyclic-di-AMP induces a memory CD4 T cell response and reduces bacterial burden following intravaginal infection with Chlamydia muridarum. bioRxiv 2024.