Antonius M. VanDongen, Associate Professor from Duke University, walks us through Arc, a new target for treating Alzheimer’s disease
Alois Alzheimer is a German psychiatrist credited with identifying the first case of the debilitating disease named after him. In 1906, he described neurofibrillary tangles and amyloid plaques in his patient’s brain as unique hallmarks of her dementia.
Advances in neuroimaging, genetics, and molecular biology have expanded our understanding of the mechanisms underlying Alzheimer’s disease (AD) significantly. But despite heroic efforts to find a cure, there is currently no therapy that prevents, stabilizes or reverses the progression of this disorder that is poised to take on epidemic proportions as the world ages.
Over 500 clinical trials for AD have failed. Ninety-eight unique compounds targeting amyloid plaques were tested in phase II and II trials, and none showed efficacy. Thirty phase III trials recruited thousands of patients and cost hundreds of millions to test drugs developed to prevent the formation or reduce existing amyloid plaques. None showed any efficacy in curtailing AD. Therefore, these neuroanatomical features discovered in 1906 appear to be the proverbial red herring.
Although both amyloid plaques and neurofibrillary tangles correlate strongly with the pathophysiology of AD, they may not be causative for the disease. The strict focus on the amyloid hypothesis for developing AD therapies, at the exclusion of other potential mechanisms, is responsible for the current lack of adequate treatment. We need to change course and pursue new avenues.
The memory gene Arc
Enter Arc: activity-regulated cytoskeleton-associated protein. The gene encoding Arc is required to create stable, long-lasting memories: mice in which this gene has been genetically silenced have intact short-term memory but cannot store memories for more than a few hours.
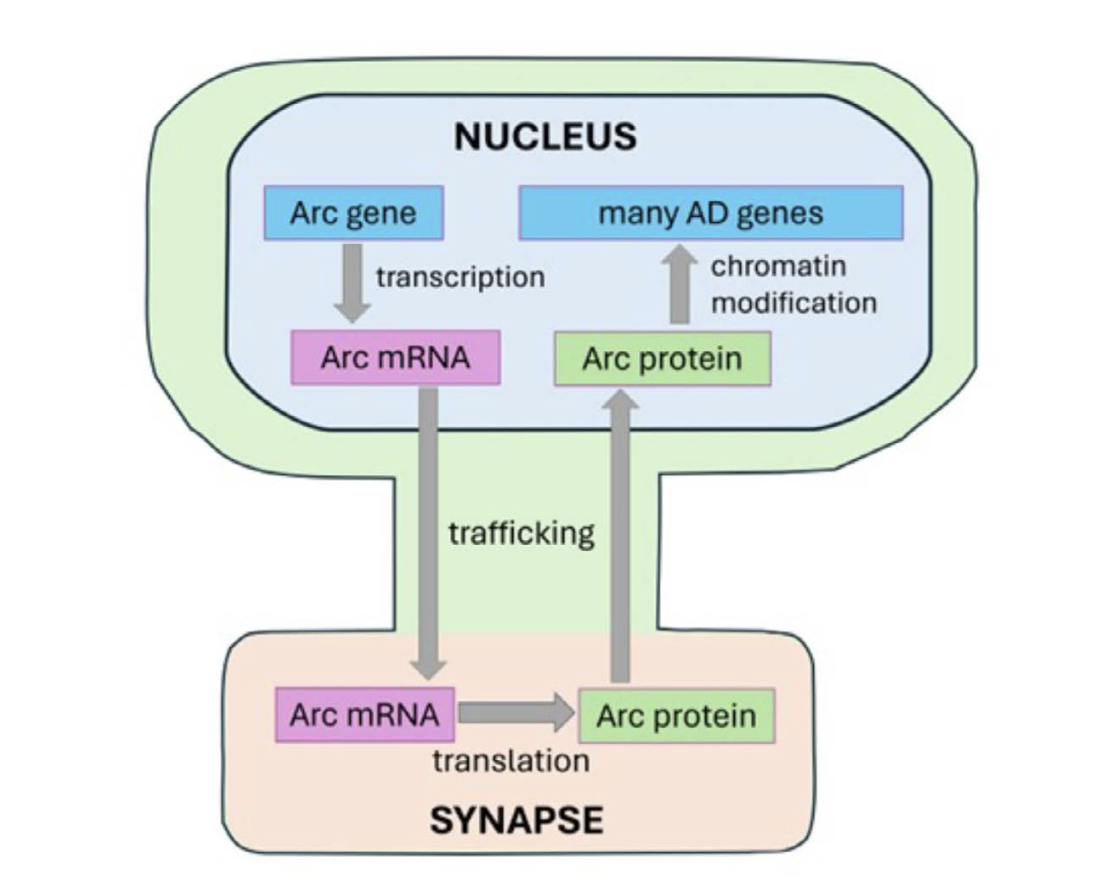
So, Arc is required for memory consolidation: the conversion of short-term fleeting memories into long-lasting, stable ones. Arc is a brain-specific protein initially found to localize to synapses, the structures comprising the connections between neurons. The functional expression of the Arc gene is strongly dependent on neuronal activity and is regulated at several levels. Transcription of the Arc gene is a sophisticated process tightly regulated by several molecular mechanisms and controlled by neuronal activity.
After Arc mRNA is generated by gene transcription, it is trafficked to synapses where it can be locally translated. Expression is also regulated at the level of protein synthesis: efficient translation of Arc mRNA requires activation of the NMDA receptor, another critical component of synaptic plasticity. It is further enhanced by dopamine and adrenergic signaling, which are associated with reward and fear pathways. This complex mechanism allows emotions to control which memories are stored by tightly controlling Arc expression in time and space. Increasing Arc expression in a subset of interconnected neurons that process a sensory input strengthens their connections, creating a memory trace.
Arc at the synapse and in the nucleus
Arc’s function has been best studied at neuronal synapses, where it was initially localized. There, it controls structure and function by regulating the number of neurotransmitter receptors in a connection and its size. However, our laboratory has identified a second location for Arc: the neuronal nucleus. There, Arc forms distinct puncta that colocalize with transcription regulation sites (PML bodies) and an epigenetic regulator of gene expression (Tip60).
High-resolution microscopy experiments show that Arc dynamically interacts with chromatin, the structure that packages the genomic DNA (movie). Chromatin structure is a crucial determinant of gene expression: “open” chromatin is amendable to gene transcription, while “closed” chromatin silences genes. Therefore, this close dynamic association between Arc and chromatin suggested that Arc may regulate gene transcription.
Arc treatment for Alzheimer’s disease
Previous work suggests Arc may be a promising new target for treating Alzheimer’s disease:
- Arc is necessary to form amyloid plaques.
- Arc protein levels are aberrantly regulated in the hippocampus of AD patients.
- Arc is locally upregulated around amyloid plaques, and
- A polymorphism in the Arc gene confers a decreased likelihood of developing AD.
Our laboratory recently discovered that Arc is also a master regulator of gene transcription and controls the expression of many genes implicated in the pathophysiology of AD (Biomedicines 10(8):1946). This finding provides a unique avenue for a novel therapeutic approach, which has come into sharp focus with our finding that two chromatin-modifying enzymes, PHF8 and Tip60, act as a dual-function epigenetic “switch” that regulates Arc gene expression. The function of these two proteins can be manipulated using small molecules, thereby allowing pharmacological control of Arc expression.
Targeting Arc through pharmacological intervention offers a novel, neuroprotective therapeutic strategy for AD, addressing the disease’s complexity by modulating multiple pathological pathways. Targeting Arc opens a new frontier of “multi-target” therapy designed to simultaneously intervene in several aspects of the disease. Because of Arc’s role in controlling the expression of multiple genes and pathways implicated in AD, it could serve as a therapeutic hub.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.