Jean C. Pfau, Ph.D. from the Center for Asbestos-Related Disease and Kinta Serve from Idaho State University, provide their perspective on asbestos, the pleural cavity, and autoimmune disease
Asbestos is well known as one of several causes of lung cancer and scarring of the lung tissue (interstitial fibrosis). However, asbestos is unique in causing several diseases of the pleural cavity, including mesothelioma, pleural plaques, and pleural fibrosis.
A look at the pleural tissues (pleura)
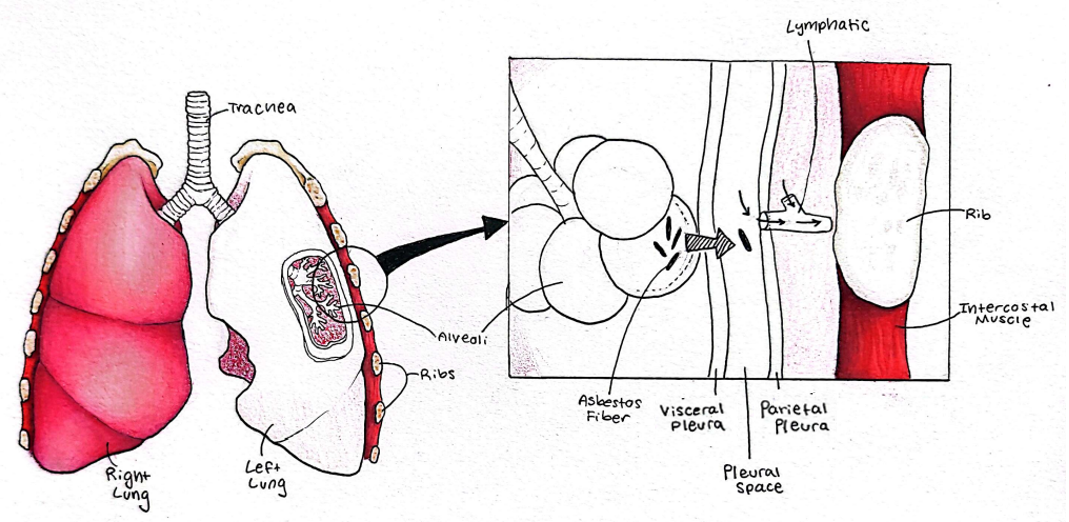
A close look at the pleural tissues (pleura) is warranted to understand asbestos pathogenesis. The pleural cavity is a very narrow, fluid-filled space formed between two thin tissues, the visceral and parietal pleural tissues (see Figure, courtesy of Saige Rigby, Idaho State University). The visceral pleura closely adheres to the surface of the lung, while the parietal pleura lines the inside of the chest cavity just inside the rib cage.
A major function of the pleural arrangement is to assist with the smooth expansion of the chest cavity during inhalation. The fluid provides a low-friction surface for movement but is also under a negative pressure, essentially a slight vacuum, snugly holding the lungs against the thoracic wall. During inhalation, we expand our chest cavity’s volume, and as long as the lungs are stuck to the chest wall, the lungs also expand, allowing air to flow in. If anything happens to prevent that vacuum, the lungs will not expand, and in fact, they will collapse.
Clearly, the pleural vacuum is critical for life, but it also creates interesting pressure gradients that facilitate the movement of inhaled asbestos fibers from the lung tissue and into the lymphatic system. (1) Lung inflammation, a key aspect of asbestos pathogenesis, increases alveolar capillary permeability (leakiness) within the lung itself, thus increasing pressures that move fibers into the pleural cavity. From there, if the fibers are small enough to fit, they can access both lymphatic and blood vessels and be carried throughout the body (Figure).
As described in a recent Open Access Government article, the average length of fibers in the pleural cavity, lymphatic tissues like lymph nodes, and other body tissues is less than 4 micrometers – so small that they can only be seen with electron microscopy. (2)
Translocation from the pleura into lymphatics occurs via tiny holes called stomata. Averaging 2-6 micrometers in diameter, pleural asbestos fibers sometimes get stuck lengthwise across the stomata and begin to accumulate, effectively blocking this translocation, especially for the smallest stomata. The presence of these fiber dams is associated with increased inflammation in the pleural cavity. (3)
Pleural tissues are composed mostly of mesothelial cells but are also populated with immune cells, namely macrophages, to provide protection from pathogens. However, the activation of these cells via translocated asbestos fibers can drive the development of inflammation and fibrosis within the pleural cavity. When macrophage cells engulf and digest foreign bodies, they send out signals to initiate immune responses; these responses are distinct depending on the type of pathogen encountered (i.e., a virus versus a bacterium).
Asbestos-related diseases arising in the pleura
However, since asbestos and similar fibers cannot be digested, their presence may result in “mixed messages” to the immune system. Such responses include the generation of harmful reactive oxygen species (ROS) and DNA damage, which can perpetuate cancers like mesothelioma, or can drive chronic, unresolved inflammation and cell death, which is linked to autoimmunity.
Cells can also deposit excess scar tissue in an attempt to seal up free fibers, thus causing pleural plaques or fibrosis. Since smaller asbestos fibers more easily translocate into the pleural cavity, it is plausible that these short fibers play a significant role in driving asbestos-related diseases arising in the pleura.
Recent work done in Dr. Serve’s lab at Idaho State University provides some of the first evidence that, after exposure into the lung airways, short asbestos fibers (mean length 1.9±2.1μm, mean width of 0.39±0.2μm) produce inflammation specifically within the pleural cavity. Using a mouse model of asbestos exposure, researchers found that exposure to short asbestos fibers resulted in a longer duration of inflammatory responses in the pleural cavity compared to the interstitial lung space.
The measured immune responses were predominated by recruited macrophages and neutrophils = innate immune cells associated with non-resolving inflammation, ROS production, and tissue damage. Thus, these responses to short fibers within the pleural cavity could potentially precipitate chronic diseases associated with asbestos, including malignant mesothelioma, pleural fibrosis, and autoimmune diseases.
The pleural cavity to understand asbestos health effects
In summary, the importance of the pleural cavity in the understanding of asbestos health effects is highlighted by the following findings:
- The pleural cavity is a key route for small asbestos fibers to interact with the lymphatics and the immune system. (4)
- Exposure to amphibole asbestos is associated with systemic autoimmune disease. (5,6)
- Markers of autoimmune disease (autoantibodies) are associated with radiographic pleural changes (7) and progressive pleural fibrosis. (8)
Thus, the pleural cavity appears to be the conduit that expands asbestos-related diseases to parts of the body outside the lung.
Funded by CDC/ATSDR 1 NU61TS000355-01-00
References
- Miserocchi, G., 2008. http://www.ehjournal.net/content/7/1/4
- Pfau, J. and K. Serve, 2024. https://doi.org/10.56367/OAG-044-11274
- Donaldson, K., et al, 2010. http://www.particleandfibretoxicology.com/content/7/1/5
- Oberdorster, G., 1988, https://doi.org/10.1093/annhyg/32.inhaled_particles_VI.149
- Diegel, R. et al, 2018. https://doi.org/10.1080/15287394.2018.1485124
- Pfau, J. et al, 2024. https://doi.org/10.1016/j.autrev.2024.103603
- Marchand, L. et al, 2012. https://doi.org/10.1016/j.toxlet.2011.10.024
- Pfau, J. et al, 2019. https://doi.org/10.1080/08958378.2019.1699616