The WATCH team, founded to elucidate the role played by specialized brain cells called tanycytes in various physiological processes, has been investigating how and where the SARS-CoV-2 virus infects the brain, and some long-term consequences of this neuro-invasion
The European Research Council (ERC) Synergy project WATCH (Well-Aging and the Tanycytic Control of Health) brings together the teams of three principal investigators: Vincent Prevot, a neuroendocrinologist working for the French National Health Research Institute (INSERM) in Lille, France; Markus Schwaninger, a neurologist at the University of Lübeck, Germany; and Ruben Nogueiras, an expert in molecular metabolism at the University of Santiago de Compostela in Spain. Together, they are researching how tanycytes – highly specialized cells residing in a tiny part of the brain called the hypothalamus – regulate various physiological processes.
An unusual suspect
In early 2020, when COVID-19 first made its appearance in Europe, it was obvious to any neurologists or neuroscientists worth their salt that the causative virus, SARS-CoV-2, must be infecting the brain, and more to the point, the hypothalamus. This part of the brain, no bigger than the end of. one’s thumb, acts as the hub through which the brain and body interact. The numerous populations of highly specialized neurons and glia that make up the hypothalamus influence almost every bodily function: growth, food intake and expenditure, including fat and sugar metabolism, water balance and kidney function, cardiovascular and respiratory function, temperature control, stress response, sexual differentiation and reproduction, and even aging, to name just a few.
Hypothalamic cells send hormonal signals to various parts of the body, principally through the pituitary gland located just under the hypothalamus, and also receive environmental information and feedback from the different parts of the body. When this communication process breaks down, diseases ensue. The hypothalamus is also functionally and anatomically connected to the nose. Perhaps most importantly, to facilitate the exchange of messages between the brain and body, the ‘blood-brain barrier’ made up of the walls of blood capillaries is porous in parts of the hypothalamus, providing easy access to a circulating virus, for example.
Within a few weeks of the emergence of COVID-19, a number of signs caught our attention: (i) neurological signs such as headaches, loss of consciousness and cognitive deficits, not to mention a dysfunctional sense of smell, (ii) the fact that men appeared to be more severely affected than women, (iii) the increased susceptibility of people with diseases of energy or fluid balance such as obesity, diabetes or hypertension, and (iv) the deregulation of processes needed to sustain life, such as detecting and responding to a lack of oxygen in the blood. Could SARS-CoV-2, a nominally respiratory virus, be capable of infecting other organs, including the brain, and particularly the hypothalamus, thus accounting for some of the more inexplicable symptoms that patients were experiencing worldwide? And if so, what would the long-term consequences of this infection be?
From acute COVID-19 to long-COVID
With the ERC’s blessing to use some of our funding, the WATCH consortium set to work: Vincent Prevot’s team focused on analysing publicly available data and, eventually, patient brains to prove the hypothalamus’s susceptibility to SARS-CoV-2, Markus Schwaninger’s team showed that cells of the blood-brain barrier were infected and killed by the virus, and Ruben Nogueiras’ team looked for viral entry molecules in the liver, which is similar to the brain in many respects, to understand why patients with certain metabolic disorders were at higher risk. However, our early results on the brain’s susceptibility, and in particular the presence of the virus in the hypothalamus of patients who died of COVID-19, were met with scepticism until other groups also started reporting neuro-invasion.
Along the way, three other significant discoveries justified our interest in the hypothalamus as a target of SARS-CoV-2 infection:
- Many COVID-19 patients develop debilitating symptoms, including neurological ones such as fatigue, headaches and ‘brain fog’, that last for months or even years after initial infection. This is known as long-COVID, post-COVID syndrome or post-acute sequelae of COVID;
- Men infected with the virus often showca drop in testosterone levels; and
- In a different project, the Prevot team showed that the decreased or deregulated production of Gonadotropin-Releasing Hormone (GnRH), the master reproductive hormone released by the hypothalamus that regulates the production of sex hormones and maturation and function of the sex organs, was the culprit behind the progressive cognitive decline occurring in patients with Down syndrome and possibly Alzheimer’s disease.
Hypothalamic GnRH neurons, in addition to their function in regulating fertility, also project to parts of the brain involved in memory, learning, and reasoning, and replacing GnRH at normally occurring doses and patterns Hypothalamic GnRH neurons, in addition to their function in regulating fertility, also project to parts of the brain involved in memory, learning, and reasoning, and replacing GnRH at normally occurring doses and patterns improved both cognitive function and intra-brain connectivity in these patients. This suggests that if the low testosterone levels shown by male COVID-19 survivors were caused by the infection or dysfunction of GnRH neurons, the same phenomenon could also explain the cognitive deficits occurring in long-COVID patients.
With the aid of several other teams in France, Spain and the UK, we also looked at COVID-19 survivors followed for months after the acute infection to ask whether and how SARS-CoV-2 was entering the hypothalamus, whether the infection altered GnRH neurons and whether the hormonal patterns displayed by male survivors of COVID-19 reflected these alterations. Our intriguing discoveries, published recently in eBioMedicine showed:
Low testosterone levels that persist in some men or that appear months or years after an acute COVID-19 infection may stem from a hypothalamic defect rather than the infection of peripheral organs. Specifically, when sex hormone levels drop, the brain compensates by releasing more GnRH to increase the levels of gonadotropins, intermediate hormones, by the pituitary, to increase sex hormone levels. In male COVID-19 patients, both in the acute phase of infection and months later, there was no compensatory increase in gonadotropins, indicating that the brain could not produce or release more GnRH.
Patients in whom testosterone levels were abnormal at delayed time points also showed dysregulation of body weight and cognitive/neurological problems: Since tanycytes, the same hypothalamic cells that regulate the release of GnRH by the neurons producing the hormone, also control the exchange of metabolic information between the rest of the body and brain circuits that control feeding, the fact that both processes are altered again suggests a hypothalamic infection. While our patient numbers were low due to the difficulties inherent in recruiting patients and conducting clinical studies during an ongoing pandemic, most of the patients with hormonal abnormalities also displayed long-COVID cognitive symptoms.
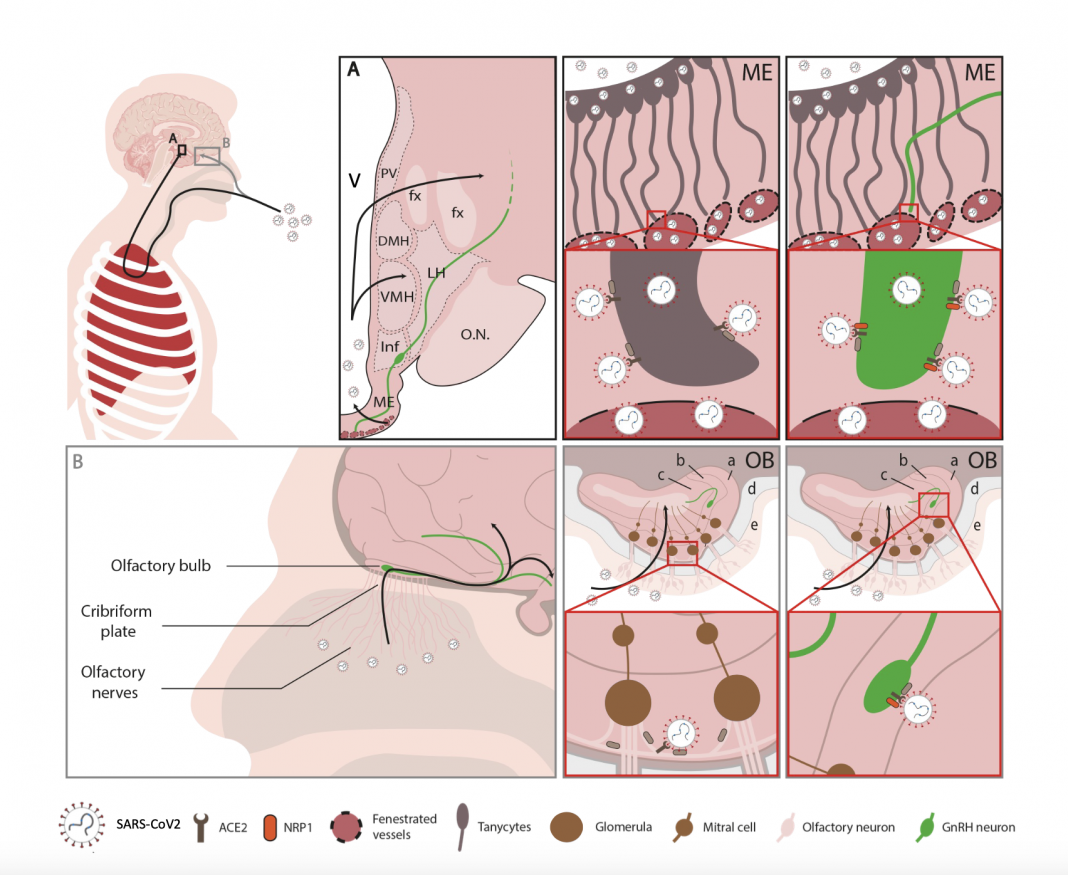
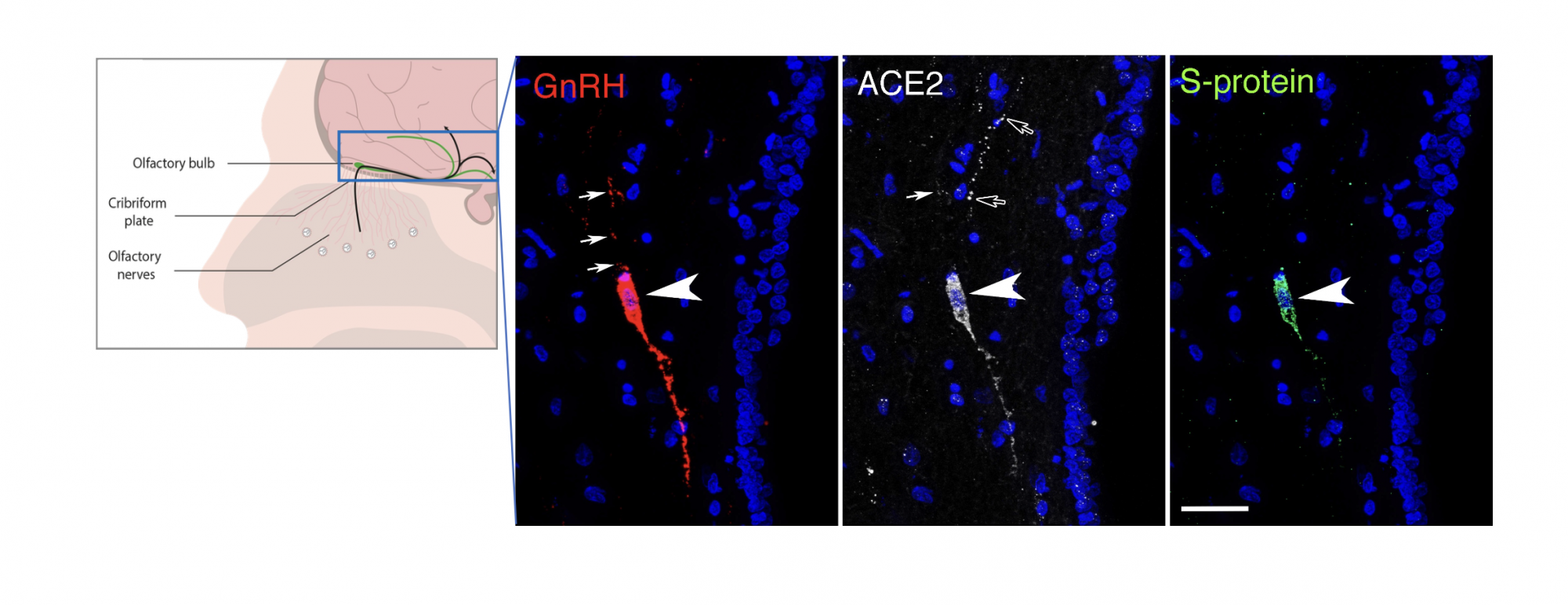
Both the hypothalamus and the nasal epithelium and olfactory bulb of the brain of patients who died of COVID-19 showed viral markers: In all the post-mortem samples available to us, viral proteins and RNA (genetic material) were detectable in these areas, including in several hypothalamic cell types, including tanycytes and GnRH neurons. Interestingly, GnRH neurons are not only in contact with the blood, into which they release their hormone, but they are born in the nose in the embryo before migrating to the hypothalamus along pathways involved in the sense of smell, and still keep one foot in the nose in adulthood, suggesting that they could have been infected through either an olfactory route or a blood-borne route.
GnRH neurons were bloated or dying in all the patient brains examined, strongly impairing GnRH production: While the adult human brain only contains around 2000 scattered GnRH neurons, most of the neurons we identified in patient samples were sick, in keeping with the drastic decrease in GnRH expression in these brains. Extrapolating from other diseases that involve a loss of GnRH in adulthood, the millions of patients worldwide who lost GnRH neurons due to COVID-19 could be at risk of increased or accelerated cognitive and metabolic deficits as they age.
GnRH neurons in fetuses and infants could be particularly susceptible to SARS-CoV-2 infection: In sections of the nose and brain of human fetuses, we identified viral susceptibility factors in newborn GnRH neurons, and also showed that the virus could infect cultured embryonic GnRH neurons. Since the normal maturation and activation of GnRH neurons is necessary not only for later reproductive function but also the development of cognitive and sensory functions by the brain, this last finding suggests an increased risk of neurodevelopmental impairments in children born to infected mothers or infected in infancy themselves.
To summarize, our work raises the spectre of a second and delayed ‘non-infectious pandemic’ of cognitive, metabolic and reproductive disorders in COVID-19 survivors. Our work has attracted other funding, a French ANRS grant, SIGNAL, to all three WATCH teams as well as two other collaborating teams to study the effects of COVID-19 on brain aging, and an ERC proof-of-concept grant, UPGRADE, to the Prevot team to study whether GnRH therapy, which has proven effective in a pilot study of Down syndrome patients, can also improve cognitive function in age-related pathologies.


This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.