Biobank Norway Project experts chart the journey from precision medicine to precision health when it comes to breast cancer prevention
Breast cancer stands as the second most frequently diagnosed cancer worldwide, with a staggering 2.3 million new cases and 685,000 deaths in 2020. (1) It is well established that cancer is a genetic disease occurring as a result of somatic accumulation of mutations in tissues with time and exposure. It is also known that 5-10% of breast cancer cases are caused by germline genetic susceptibility. (2) How these two worlds are interconnected is less studied and understood.
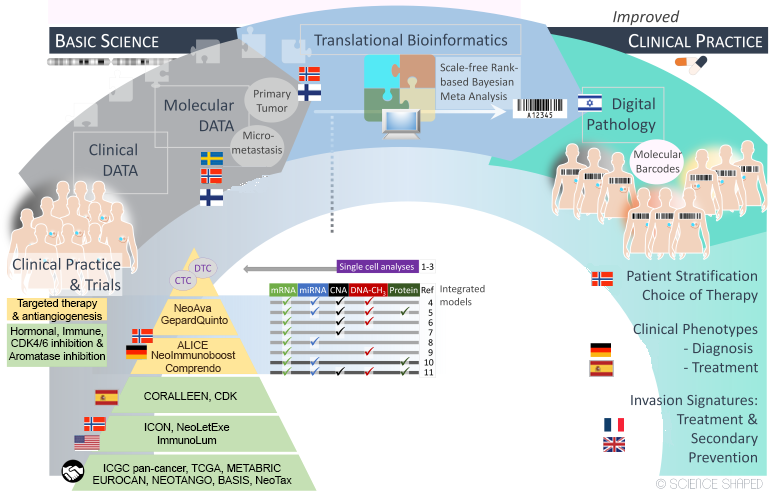
Our Horizon 2020 project, RESCUER, gathers interdisciplinary expertise in the fields of surgery, pathology, oncology, molecular biology, bioinformatics, philosophy, mathematics, and statistics. The RESCUER Consortium includes a multidisciplinary combination of partners – clinical, scientific, technical, and industry – that correspondingly express diverse and multi-faceted exploitation interests aimed to bring results to actual use in several different areas, generating broader impact. The goal is to identify the best treatment for each patient based on molecular profiling in clinical trials (Figure 1) according to the principles of precision medicine.
The above-described approach is often applied to patients with already quite advanced diagnoses. Is it possible to extend this approach to intervene earlier and prevent or postpone the disease by monitoring disease risk and environmental and lifestyle factors? Genetic factors include rare monogenic pathogenic variants (MPVs) in high and moderate-risk cancer predisposition genes such as BRCA1 and BRCA2 or CHEK2, having effects large enough to warrant monogenic testing. However, only a fraction of breast cancer cases are caused by these rare MPVs. (3)
Breast cancer risk variation
A considerable part of breast cancer risk variation is explained by low-risk variants or single-nucleotide polymorphisms (SNPs), that are common in the population. Although single SNPs have less apparent effects on the phenotype (2), they are estimated to contribute to a great proportion of missing heritability. (4) These risk SNPs are nominated according to case-control population frequency-based Genome Wide Association Studies (GWAS). Despite their estimated contribution, individual SNPs are undervalued in clinical practice due to their small effects and GWAS vulnerabilities, such as sample size, statistical power, and ethnic-specific allele frequencies.
Using GWAS, we in the Breast Cancer Association Consortium (BCAC) identified >210 low-risk loci in breast cancer, accounting for 18.3% of breast cancer familial risk. (3,5) It is suggested that GWAS associations are likely residing on Linkage Disequilibrium (LD) blocks accounted for within the GWAS design. (6) In an effort to hunt for new causal regions, we in BCAC performed fine- mapping, revealing new candidate variants, but again explored their plausibility to confer risk as single factors. Primary breast cancer prevention includes lifestyle modifications such as maintaining a healthy diet, controlling weight, engaging in regular exercise, and limiting alcohol intake.
We need precision prevention and precision health
For asymptomatic women at average risk, the European Commission Initiative on Breast and Colorectal Cancer recommends beginning breast cancer screening at age 45. (7) However, this approach does not account for the wide variation in individual women’s risks. It disregards younger women with a higher risk, but also women older than 45 with higher risk levels who could benefit from intensified screening. With the decreasing age of onset, the traditional one-size-fits-all approach of breast cancer screening does not account for individual variations in risk factors such as genetic predisposition, family history, breast density, and lifestyle. Therefore, we need precision prevention and precision health. Risk-stratified screening aims to tailor screening strategies to individual risk profiles, enhancing patient benefits while minimising harms, and has been proposed as an alternative to age-based screening. (8)
In 2019, Mavaddat et al., demonstrated the clinical utility of SNPs by developing the polygenic risk score (PRS), an overall measure of an individual’s breast cancer risk according to its genetic profile on 313 low-risk SNPs. Variants of this model represent some of the strongest associations identified in breast cancer GWAS. Therefore, genetic predisposition measured by PRS can be an essential component in risk-based, personalised, breast cancer prevention. Since then, we and others have explored the ability of this prediction tool to enable population stratification based on breast cancer risk (9) and the personalised management of individual risk. (10)
The potential of PRSs in healthcare
The potential of PRSs in healthcare has been extensively studied and demonstrated in breast cancer. Still, currently, there is yet to be a broad consensus on the clinical indications for its use, nor are there established guidelines for its implementation. This avenue has a direct clinical impact by facilitating the earlier and broader prevention of the disease in the short term for all female individuals, irrespective of age or family history. Given that prevention is one of the fundamental actions in reducing disease morbidity, personalised risk prediction based on genetic factors holds the potential to improve breast cancer survival in the long term.
Together with the Oslo Cancer Cluster, UiO, OUS, Vestre-Viken and Antegenes, a SME from Estonia, we have been active in breast cancer PRS research and development, combining an overview of the evidence base with expert opinion for indications for clinical use. Based on data we have generated, as well as from various other studies and existing breast cancer prevention and screening services, we will explore the indications for clinical use of breast cancer PRS in 1. Management of cancer-free women with a family history of cancer. 2. Individual personalised breast cancer prevention and screening in healthcare. 3. Breast cancer screening programs for more personalised screening. PRS testing can be a new standard component for breast cancer risk assessment in personalised risk-based breast cancer prevention. New approaches for combining SNPs in PRS should be devised.
The PRS model will be assessed for its performance using data from our Norwegian breast cancer study (NBCS) subset (discovery cohort) and the Biobank Norway and UK BioBANK (validation cohorts). The NBCS dataset also includes lifestyle parameters, epidemiological data, tumour characteristics, and clinical parameters, which will be used in the determination of PRS associations (313 model and enhanced model) to clinical parameters. Additional validation and exploration of phenotypic and genotypic correlations can be obtained by incorporating the UK Biobank data, a large-scale prospective study that includes extensive genetic and phenotypic information from approximately 500,000 participants. This dataset provides an additional rich resource for validating our findings across a diverse and large population.
Enhance our understanding of breast cancer risk
The UK Biobank’s extensive phenotypic data enables a comprehensive assessment of the relationship between genetic variants and a wide range of traits. This will enhance our understanding of the multifactorial nature of breast cancer risk. For each individual being diagnosed with a new actionable pathogenic variant, it is imperative to obtain information regarding the risk of developing cancer and the sensitivity to therapy modalities. This will have a strong impact on the management of disease, follow-up and potential treatment.
It will allow “previvors” to actively prevent disease and carriers to plan families, which is of clinical importance for stakeholders and patients. Being able to assess risk and intervene with prevention will open the avenues to transform precision medicine into precision health.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.