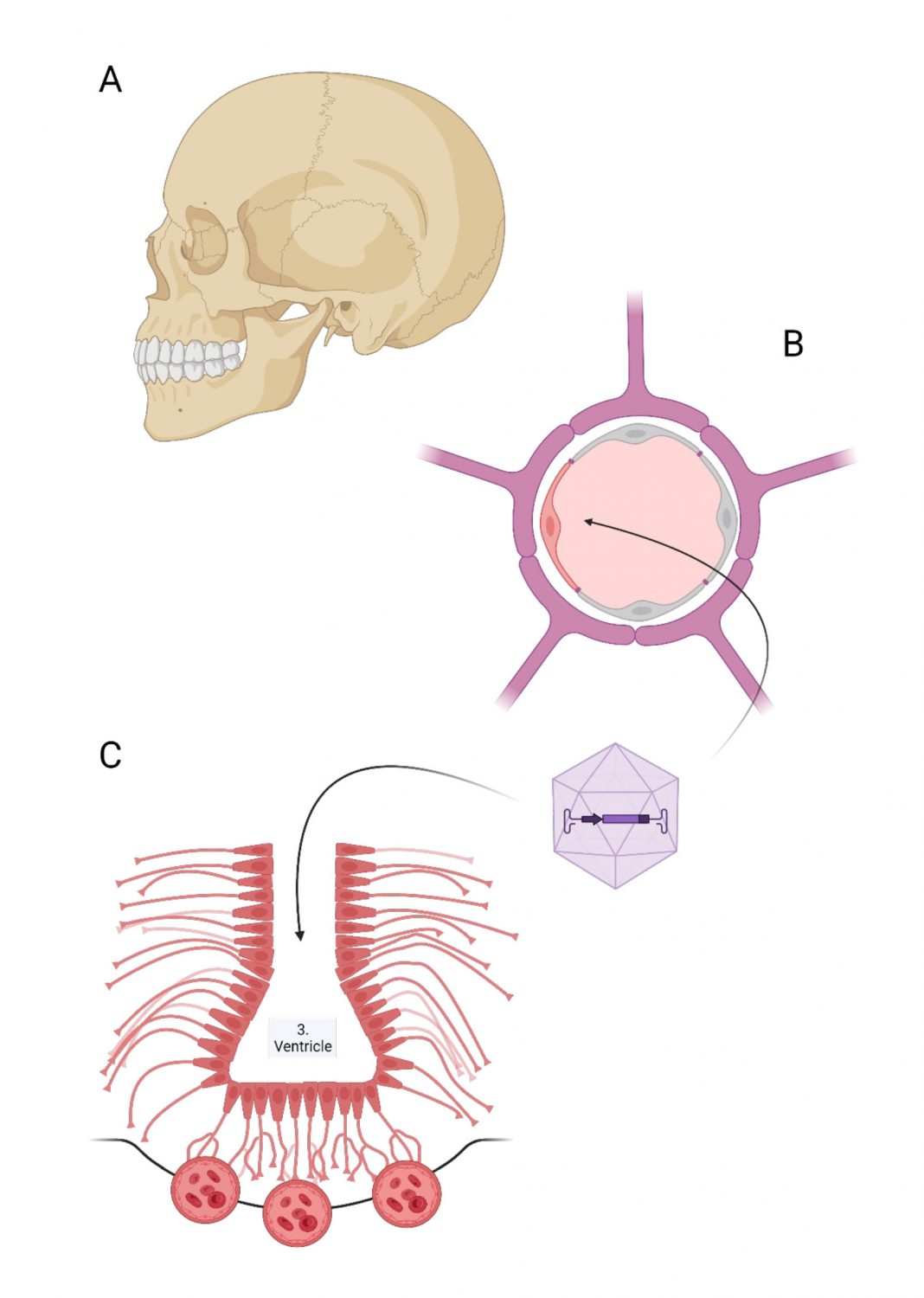
Reflecting on the challenges in treating brain diseases, this article explores ways to transduce the blood-brain barrier as well as the critical role of tanycytes as a target for gene therapy vectors
It is vital that the precious central nervous system is protected from exogenous harmful factors that can damage it. As an innate helmet, the skull protects the brain from mechanical trauma. The blood-brain barrier, on the other hand, serves as a much less conspicuous shield that prevents molecular injury from taking place.
Essentially, it comprises the wall of blood vessels that serve as the gatekeepers of the brain, the Holy Grail of cognition and personality. Only substances with lipophilic properties, such as ethanol, have unrestricted access to the brain. Due to the blood-brain barrier, most biologically active molecules of a more hydrophilic nature are excluded from the central nervous system (CNS). Several drug candidates share this fate. This makes the blood- brain barrier one of the most significant obstacles to developing novel treatment strategies for brain diseases. In particular, genetic diseases are difficult to treat because high-molecular vectors carrying therapeutic genes cannot cross the blood-brain barrier. In this situation, we propose the alternative strategy that gene vectors need not overcome the blood-brain barrier but directly transduce the barrier, thereby making the barrier a place where missing genes can be expressed.
The success of this strategy has been tested in a model of inherited MCT8 deficiency. MCT8 is a transporter for thyroid hormones in the blood-brain barrier. Genetic defects in MCT8 (Allan-Herndon-Dudley syndrome) impair MCT8 transport function. In this disease, thyroid hormones cannot reach the brain from the thyroid gland. The lack of thyroid hormones in the brain leads to severe intellectual disability in children with genetic MCT8 deficiency. To restore MCT8 function, we used an adeno-associated virus (AAV) to transduce brain endothelial cells with the MCT8 gene (Figure 1). This intervention elevated thyroid hormone levels in the brain and markedly improved neurochemical parameters and neurological function (gene therapy targeting the blood–brain barrier).
To make this treatment strategy work in other diseases affecting the blood-brain barrier, we optimized the gene vector by including gene regulatory elements that increase the specificity for the blood- brain barrier (DNA sequences to target brain endothelial cells). The approach has two significant advantages.
First, gene vectors can be administered intravenously, and second, they reach all parts of the central nervous system, increasing their translational potential. However, the notable limitation is that it targets exclusively the endothelial blood-brain barrier, a large interface between blood circulation and CNS extending over an area of 12-18m2 in the human brain. (1)
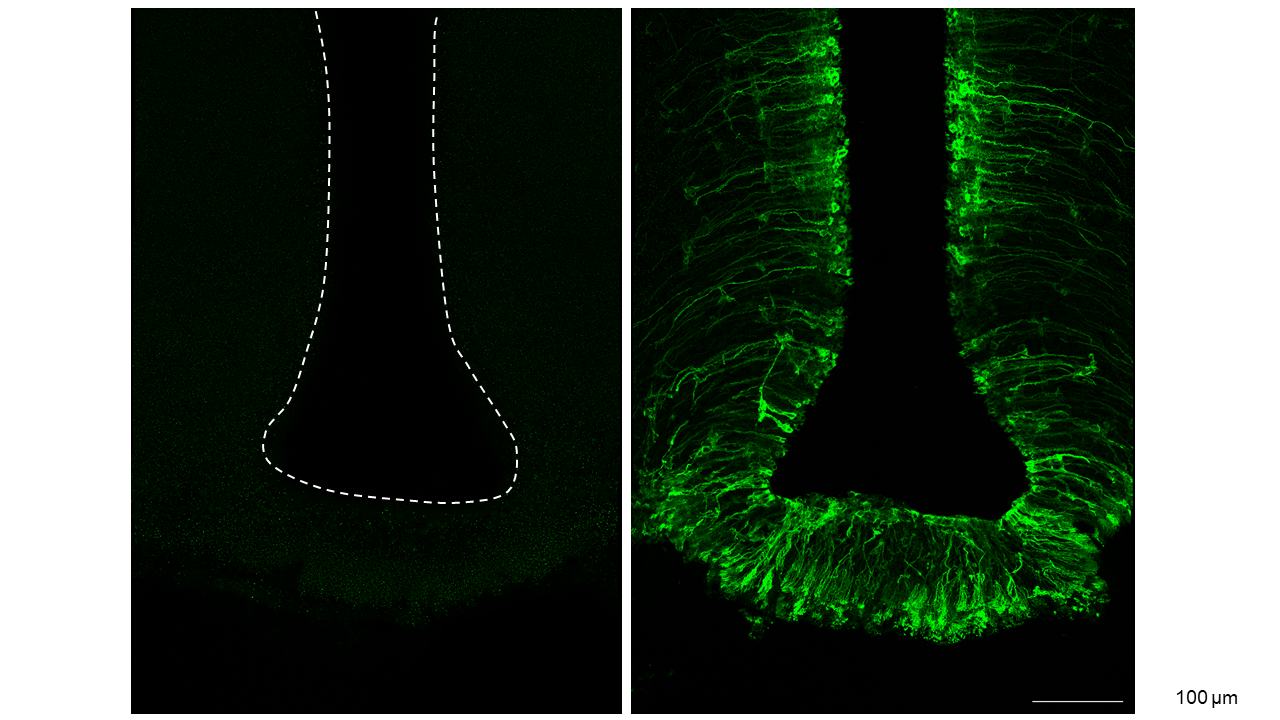
The role of tanycytes in gene therapy
In specialized brain areas, mainly in the hypothalamus, not vessels but glial tanycytes form the blood-brain barrier. Although the tanycytic blood-brain barrier is much smaller in area, it is just as critical for the metabolic and endocrine functions of the brain as the endothelial blood- brain barrier. Tanycytes form a tight barrier between blood and the hypothalamus, preventing unspecific diffusion of compounds. However, on the other hand, they selectively transport essential mediators or nutrients into the CNS.
Our consortium’s recent work has revealed key functions of the tanycytic blood-brain barrier in body weight regulation and energy expenditure.
For instance, tanycytes send signals from the periphery to the brain to reduce feeding and increase energy expenditure in cases of obesity when fat deposits are filled. (Leptin brain entry via a tanycytic LepR). They also mediate the effects of prolonged breastfeeding on metabolic centres in the brain (prolonged breastfeeding protects from obesity).
Given these key gatekeeper functions of tanycytes, it is intriguing that we could develop an AAV-based strategy to target this cell type with gene vectors (tanycytes control the hormonal output of the hypothalamic-pituitary-thyroid axis | Nature Communications) (Figure 2). Further optimization of this approach could be the basis for future tailored therapies for metabolic or endocrine diseases.


This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.