A living biobank can generate new insights about our care; Francois Lamontagne, Paul Hebert, and Michelle Kho tell us more
A detailed examination of diseased tissues and other biological specimens has enabled a better understanding of heart diseases, tumors, and syndromes such as sepsis. Biobanking involves collecting, storing, cataloguing and eventually analyzing these specimens. (1, 2) Specimens are most valuable when they are systematically linked to accurate clinical information (i.e. data), including risk factors, disease trajectories and ultimately, outcomes that are important to patients. This is no small feat and requires a lot of planning and infrastructure. One of the most important and difficult aspects is working with clinical teams and services to obtain access to patients and their medical records.
Success stories of biobanks and their impact
Like other Western countries, Canada has many small and occasionally larger biobanks distributed throughout many academic institutions. One of Canada’s most successful investments in biobanks includes the Canadian Partnership for Tomorrow’s Health (CanPath), which includes multiple regional biobanks (e.g., Ontario Health Study, Alberta tomorrow project, BC Generations Project, Atlantic PATH, CARTaGENE) and studies genetic, environmental, and lifestyle factors that influence the risk of developing illnesses in the general population. (3, 4) Other successful biobanks, such as the Canadian Tissue Repository Network (CTRNet), focus on specific illnesses like cancer with the hope that careful analysis of data and biological specimens donated by patients will help improve care by finding new cures and diagnostic tests. Arguably, the most successful biobank in the world is the UK Biobank. For example, we owe much of our current understanding of COVID-19 pathophysiology to the data and biological samples from half a million United Kingdom participants stored in this biobank. (5, 6)
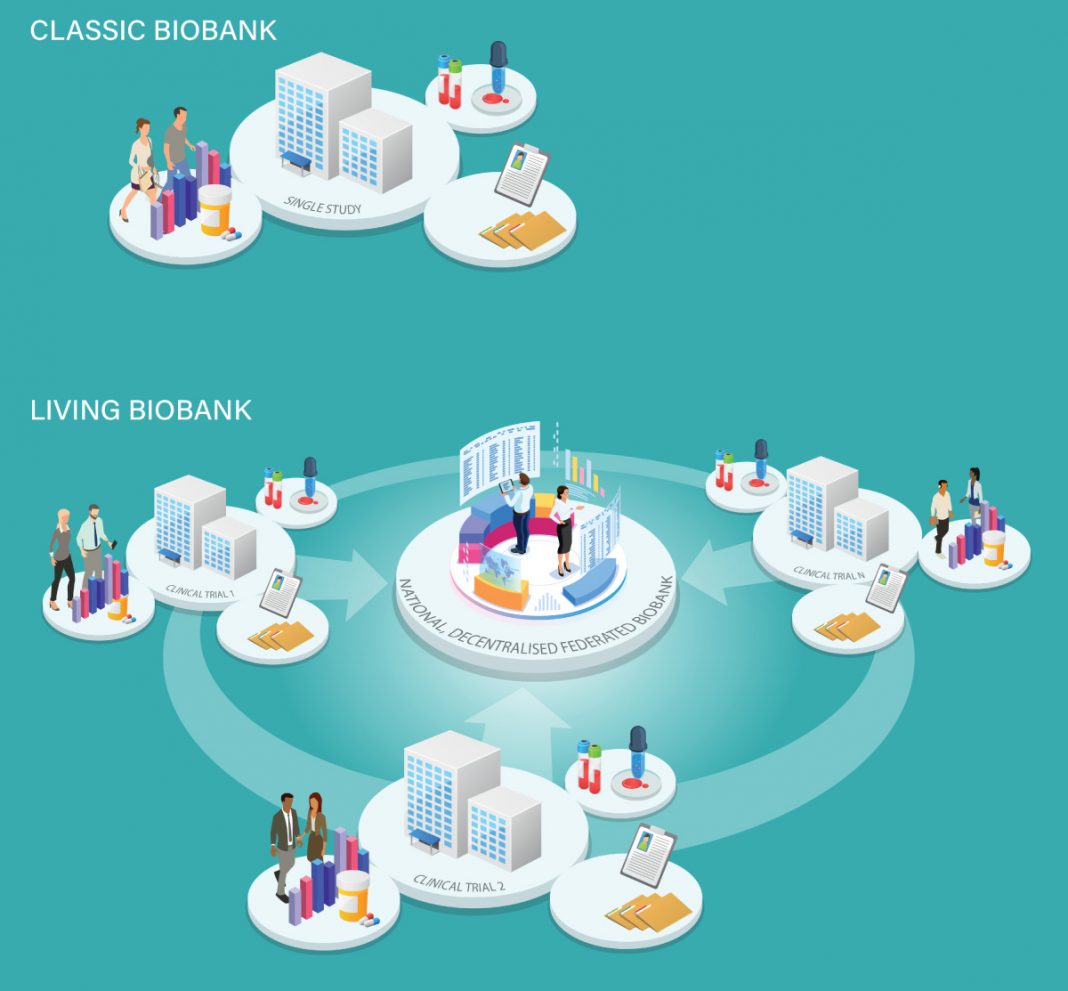
Why ‘classic’ biobanking is neither enough nor sustainable
As important as this is, it is not sufficient. We have used biobanks well to study certain diseases, but many health conditions remain understudied. One reason for this is that creating and maintaining high-impact biobanks is prohibitively expensive. According to a 2022 report by KPMG, the UK Biobank had cost in excess of £180m since its inception. At first glance, it is easy to attribute these costs to the ‘hard infrastructure’ required to store and analyze the samples, such as freezers and vials. However, a freezer filled with samples is not a biobank. A main driver of the biobank-related costs is the ‘soft infrastructure’ – i.e., the workforce and processes required to recruit participants, obtain informed consent, collect data and specimens, etc. Unfortunately, in Canada, such research activities are not considered part of routine care and are very difficult to undertake in most clinical environments. Moreover, such costs increase incrementally for as long as biobanks continue to enroll new participants due to the ever-growing number of participants, which must be followed over time. Accordingly, many biobanks either fail to collect adequate data, suffer significant cohort attrition due to loss to follow-up, or close when funding runs out, resulting in considerable waste of resources.
A low-cost opportunity to build a sustainable, high-impact, and ‘living’ biobank infrastructure
Besides biobanks, other studies also use a similar ‘soft infrastructure.’ For example, clinical trials (i.e., studies that allow researchers to compare one treatment with another) also search for eligible participants, obtain informed consent, and collect data and samples. Unfortunately, research funders tend to fund biobanks and other health studies separately. This siloed approach represents a huge missed opportunity because the same tasks are funded twice. Moreover, launching parallel projects creates competition instead of incentivizing what could be a mutually beneficial process. Clinician scientists who lead clinical trials generally excel at finding eligible participants, collecting high-quality data, and coordinating follow-up. Moreover, because we will continue to fund clinical trials to evaluate therapies, these studies jointly represent a never-ending source of high-quality data and samples that could feed into a nationwide, decentralized, federated biobank available for ambitious basic science initiatives. Increasing access to valuable clinical trial data and specimens would significantly enhance Canada’s biobanking capacity in a way that would be sustainable because most of the ‘soft infrastructure’ costs would be covered by clinical trial funding.
It would also evolve in parallel with the clinical trial portfolio and reflect changes in health research priorities. In hindsight, instead of funding biobanks dedicated to COVID-19 that have already lost much of their relevance, it would have been possible to take advantage of the infrastructure of a long list of already funded COVID-19 studies. Implementing this model in the future will require a very different clinical research paradigm.
A clear way forward for a ‘living’ biobank infrastructure
Some of the steps that lead to a sustainable, high-impact, decentralized, federated, and ‘living’ biobank infrastructure include:
- Clinician scientists funded to conduct clinical trials should systematically seek consent from eligible participants for future uses of data and biological specimens in the context of a collective biobank effort;
- Study data and a fraction of the biological specimens should be made available to basic scientists following a clear and rigorous governance framework;
- Laboratories with the necessary expertise available and accountable for storing and monitoring and analyzing specimens should be identified as potential biobank hubs;
- Basic science teams granted permission to analyze specimens and data should make the results of their analyses available to clinician scientists and the research community, thereby enriching the knowledge derived from the original studies.
This is on us
In essence, we describe a model that would aim to make clinical research outputs – data and specimens collected from human patients – findable, accessible, interoperable, and reusable. (7) The FAIR principles are not new, but they are not widely applied, and we, health scientists, are collectively responsible for this. Fortunately, this also means that we can fix this. In the age of artificial intelligence, there is still a need for human common sense.
References
- Shaw D, Elger B, Colledge F. What is a biobank? Differing definitions among biobank stakeholders. Clinical genetics 2014;85(3):223-227.
- Biobank U. UK Biobank ethics and governance framework. UK BIOBANK 2007;3.
- Borugian MJ, Robson P, Fortier I, et al. The Canadian Partnership for Tomorrow Project: building a pan-Canadian research platform for disease prevention. CMAJ 2010;182(11):1197-201. DOI: https://doi.org/10.1503/cmaj.091540.
- Dummer TJB, Awadalla P, Boileau C, et al. The Canadian Partnership for Tomorrow Project: a pan-Canadian platform for research on chronic disease prevention. CMAJ 2018;190(23):E710-E717. DOI: https://doi.org/10.1503/cmaj.170292.
- Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022;604(7907):697-707.
6. Kousathanas A, Pairo-Castineira E, Rawlik K, et al. Whole-genome sequencing reveals host factors underlying critical COVID-19. Nature 2022;607(7917):97-103. DOI: https://doi.org/10.1038/s41586-022-04576-6.
7. Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016;3:160018. DOI: https://doi.org/10.1038/sdata.2016.18.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.