Professor Faith Osier, Director of the Chanjo Hub at Imperial College London, shares her vision for vaccines and biologics manufacturing in Africa to secure lives and livelihoods and drive economic growth
The COVID-19 pandemic demonstrated that Africa should not rely on donations from rich countries. Africa has the highest burden of endemic infectious diseases globally, accounting for >227mn years of health life lost, an annual productivity loss of >$800bn and an infection-related mortality of ~10m/ year. The list of diseases is long, diverse and challenging. It includes infectious diseases like cholera, Ebola, meningitis, measles, yellow fever, mpox and COVID-19, to mention a few. Some are caused by zoonotic pathogens that spill over from animals to humans. The need for local capacity to rapidly respond to known and unknown threats and to mitigate global impacts is urgent.
Africa can manufacture her own vaccines
The Africa Centres for Disease Control and Prevention (Africa CDC) and the African Union (AU) have called for a New Public Health Order with the objective that Africa manufacture 60% of its vaccine needs by 2040, up from 1%. Progress towards this ambitious goal will require not only the requisite technical skills and technologies but also the simultaneous strengthening of the ‘vaccine manufacturing ecosystem in Africa’ for long-term sustainability. The Africa CDC Platform for Harmonized African Health Products Manufacturing (PHAHM) has laid out a framework for action, convening continental and global stakeholders.
Vaccines and biologics: Challenges must be overcome
Vaccine manufacturing is a lengthy process that is highly complex and stringently regulated; it is also very expensive. The diversity of organisms and disease challenges we face means that ‘no size fits all’. A range of vaccine manufacturing platforms and technologies are continuously being developed to counter this diversity. Which of these are best suited for Africa? Is technology and ‘know-how’ the major problem? Vaccine manufacturers in Africa grapple with multiple major long-term and interconnected challenges. Is the enterprise economically viable? Can the market be guaranteed? Does the necessary workforce exist, and can it be maintained? Will Africans buy vaccines ‘made in Africa’? Will their governments prioritise ‘home-made’ products? Is the regulatory infrastructure supportive?
A bottom-up approach
Chanjo is an African-led academic-industry hub that is researching, developing, and implementing a bottom-up approach to establishing local vaccine manufacturing for endemic diseases of importance to Africa. We focus on end-to-end vaccine manufacturing in African countries using a range of low-cost technologies that are practical and well-suited to local realities. We deliberately identify and support new and emerging African manufacturers in Africa and the African diaspora. We leverage existing local infrastructure, harness international and local partnerships, raise funding, facilitate technology transfer, provide training and support product development. We start small, beginning with the manufacturing of small batches of clinical trial lots for new vaccines and biologics. This builds the necessary muscles, wisdom and skill, even as the African vaccine manufacturing ecosystem develops and matures.
Embracing low-cost technologies
Our product pipeline includes malaria, COVID-19, rabies, Rift Valley fever, avian influenza, African horse sickness, and biosimilar monoclonal antibodies.
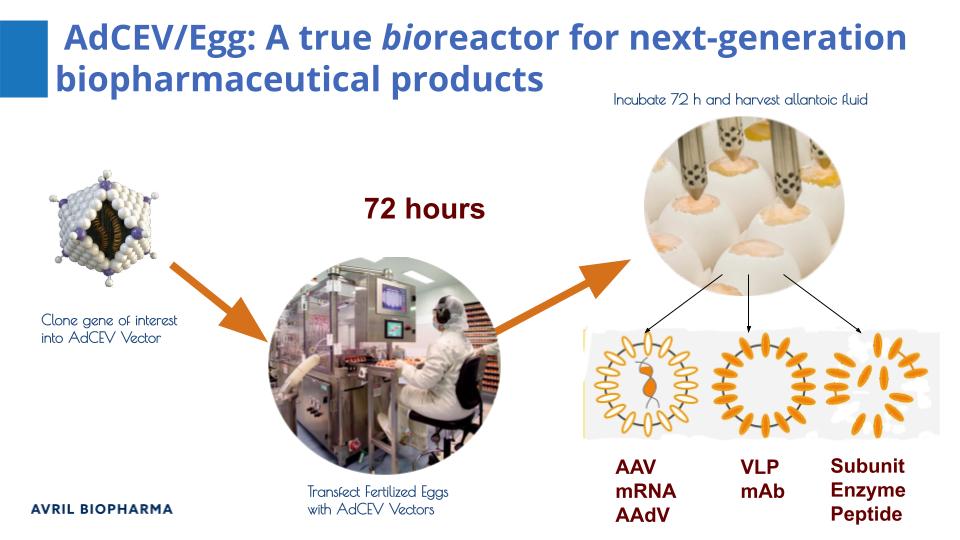
‘Make your vaccine in an egg’
Avril Biopharma Inc. is an African-owned Contract Development and Manufacturing Organization (CDMO) specialising in technologies and enterprise business models for low-cost, agriculture-based biomanufacturing. It designs, tests and scales recombinant products using structural genetics computing, AdCEV vector protein expression in fertile hen eggs, and EggPharm modular manufacturing enterprises. It is partnering with the Animal and Water Research Institutes at the Council for Scientific & Industrial Research (CSIR) in Accra, Ghana to manufacture subunit vaccines in eggs.
‘Make your vaccine using mRNA’
The UK Centre for Process Innovation supports the design, development, optimisation, and demonstration of bespoke manufacturing processes specific for biologic products, including mRNA vaccines. It is ISO 9001 certified for vaccine scale-up and manufacturing. It is partnering with TASA Pharma (TP) to manufacture subunit vaccines using mRNA technology. TP founded East and Central Africa’s first sterile manufacturing facility and supplies GMP-grade intravenous products in Kenya and regionally. TP is closely aligned with the Kenya Biovax Institute (KBI), the government-mandated institute for vaccine manufacture, and is designated under the WHO mRNA technology transfer program.
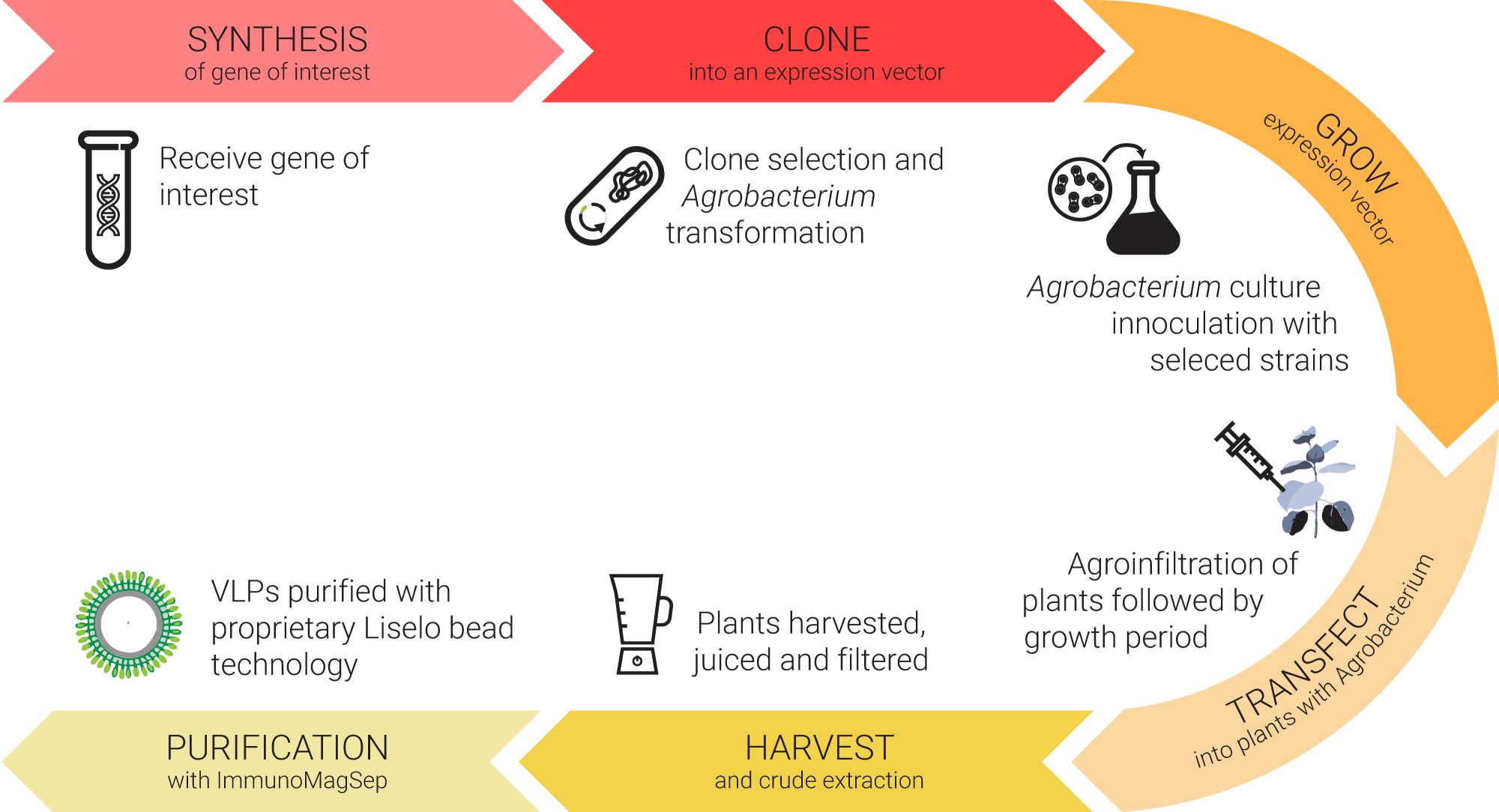
‘Make your vaccine in plants’
Liselo Labs (LL) is an African research and development company focused on One Health-centric management of disease in animals and humans, with capacity and experience in the development, clinical evaluation, and commercial-scale manufacture of immunotherapeutics and vaccines. In partnership with the University of Cape Town and the Council for Scientific Industrial Research, South Africa, LL is advancing the development of a range of vaccines and biologics manufactured in Nicotiana Benthamiana plants. The process can be scaled massively, rapidly, and relatively inexpensively.
The Imperial Chanjo Hub is funded by the Department of Health and Social Care as part of the UK Vaccine Network (UKVN), a UK Aid programme to develop vaccines for diseases with epidemic potential in low and middle-income countries (LMICs). The Institute of Infection drives interdisciplinary research. FO is supported in part by the NIHR Imperial Biomedical Research Centre.


This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.