Martin Van Den Berghe, CEO of Cytochrome, discusses catalyzing mineral weathering for permanent, safe, and cost-effective carbon storage
A self-regulating planet
Earth is unique as it is the only planet we know that harbours life. The geological record informs us that both life and liquid water have been inalienable components of our planet for over 3 billion years. Yet three billion years ago, the sun was emitting only about 75% of its current solar radiation, which was not enough heat to sustain liquid water under present atmospheric conditions. Earth was able to sustain liquid water through a very strong greenhouse effect from very high atmospheric CO2 concentrations. Over the eons the sun gradually got brighter and hotter, and atmospheric CO2 concentrations, in turn, gradually decreased through continuous weathering. (1, 2)
Mineral weathering is a natural chemical reaction in which CO2 in the atmosphere reacts with common rock-forming minerals and transforms into bicarbonate, a very stable and inert salt similar to baking soda, accumulates in the oceans (equation 1).
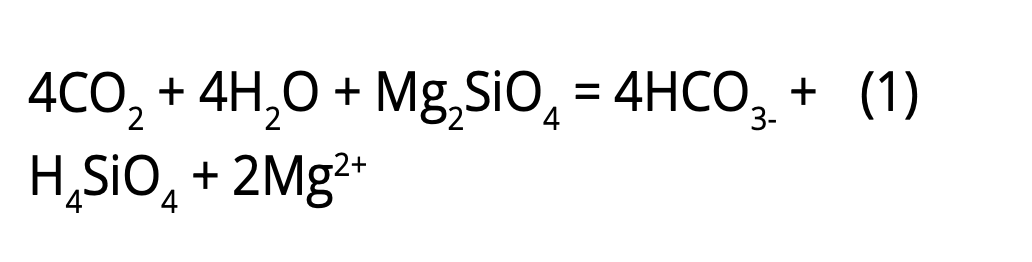
On a global scale, this process is self-regulating, as it is a chemical process sensitive to temperature and CO2 concentrations.(3) High CO2 contents in the atmosphere combined with high temperatures will promote rapid weathering reaction rates, thus limiting the amount of CO2 that can accumulate in the atmosphere and removing the risks of a runaway greenhouse effect. On the other hand, low CO2 content and low temperatures will decrease weathering rates, thus avoiding the risks of the planet becoming too cold and completely frozen. With this process, the Earth can also regulate other climate forcings, such as CO2 inputs from volcanic activity or decreases from photosynthesis.
With mineral weathering, our planet maintains a delicately balanced relationship between geology, chemistry, and biology, ensuring that life can continue to thrive for billions of years despite very significant changes in solar, geological, and atmospheric conditions. This phenomenon is often called “Earth’s thermostat” and is scientifically very well-established and uncontroversial.
Our changing climate
With this high-level scientific understanding of how our planet works, one might feel inclined to conclude that current anthropogenic climate change, and all the challenges that come with it, can be corrected by no other than natural processes alone. This is unfortunately inaccurate as (i) the current rate of climate change far exceeds the natural rates of mineral weathering, and (ii) mineral weathering keeps the Earth in a livable envelope, but fluctuations in climate still bring along with them major stresses on ecosystems.
Under present conditions, the natural rates of mineral weathering, and the associated CO2 sequestration rates, equate to ~3 % of annual global emissions from human activities. (4) Thus, natural weathering rates work well over time scales of centuries – millennia but are too slow to address our current emission rates. Indeed, the geological record further informs us about past climate change events, during which Earth’s climate has remained livable, but not without major disruptions to global ecosystems, including major extinction events. (5, 6)
Climate change events bring with them profound changes to glaciation regions, erosion patterns, weather patterns and global sea levels that can wreak havoc on freshwater availability, agricultural production, and overall, our ability to feed billions of people and sustain coastal cities and infrastructure. Climate change may not threaten life on Earth, but it absolutely has the potential to bring devastating impacts on our global civilization and prosperity.
Enhancing mineral weathering with microbes
Several strategies have been proposed to enhance mineral weathering to address climate change. Most have focused on spreading minerals of specific grain sizes in open environments such as beaches or agricultural soils as a means to accelerate their weathering. While this approach can benefit from synergies with existing industries such as agriculture and coastal engineering, weathering rates are still dependent on uncontrolled, natural processes and can’t remain elevated over years – decades due to increasingly well- documented secondary reactions inhibiting their chemical reactivity with CO2. (7)
However, recent studies have shown that microbes like bacteria and fungi have a variety of mechanisms to remove such inhibitors and further enhance mineral weathering rates sustainably. One of these mechanisms is through the production of ligands such as siderophores, compounds produced by microbes to capture metals like iron in the natural environment and enable them to access them as nutrients. In other words, some microbes have the ability to “eat” certain metals directly out of minerals and acquire the nutrients they need to grow. This process, in turn, has been shown to accelerate mineral weathering by an order of magnitude. (8, 9)
Other processes involve the attachment of fungi or bacterial biofilms directly onto the surface of minerals, leading to very high concentrations of organic acids along the mineral surface, and have also been shown to increase mineral weathering rates by an order of magnitude in a sustained way. (10-12)
Leveraging microbial growth to catalyze specific chemical processes is by no means new, as global-scale industries have successfully utilized microbes in the biochemical and medical industries, the mining industry for both metals extraction and environmental remediation, not to mention the alcoholic beverage industry (13, 14).
Furthermore, advances in genetic engineering now enable scientists to enhance very targeted microbial processes, specifically siderophore synthesis or biofilm growth, in such a way as to further supercharge these naturally occurring traits and further enhance desired effects, in this case, mineral weathering rates (9, 10).
In short, for mineral weathering to become a highly effective strategy to address our current anthropogenic climate change, we need to increase natural weathering rates by a factor of ~35. Several well-known microbial processes can increase dissolution rates by a factor of 8-10. By simply combining these processes, augmenting them with genetic engineering techniques, or even adding them to existing enhanced weathering technologies such as Cytochrome’s, microbial catalysis has the potential to propel mineral weathering as the single most cost-effective strategy to permanently sequester CO2. (15)
Enhanced weathering efforts are, however, operating as startups in a challenging financial environment and require better support to demonstrate their full potential, particularly with the scaling-up, permitting and industrial integration process. Such support will greatly enable meeting net-zero 2050 goals.
References
- C. Sagan, G. Mullen, Earth and Mars: Evolution of Atmospheres and Surface Temperatures. Science 177, 52–56 (1972).
- J. E. Lovelock, A. J. Watson, The regulation of carbon dioxide and climate: Gaia or geochemistry. Planet. Space Sci. 30, 795–802 (1982).
- J. D. Rimstidt, S. L. Brantley, A. A. Olsen, Systematic review of forsterite dissolution rate data. Geochim. Cosmochim. Acta 99, 159–178 (2012).
- L. L. Taylor, J. Quirk, R. M. S. Thorley, P. A. Kharecha, J. Hansen, A. Ridgwell, M. R. Lomas, S. A. Banwart, D. J. Beerling, Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 6, 402–406 (2016).
- J. L. Blois, P. L. Zarnetske, M. C. Fitzpatrick, S. Finnegan, Climate Change and the Past, Present, and Future of Biotic Interactions. Science 341, 499–504 (2013).
- M. C. Urban, Climate change extinctions. Science
386, 1123–1128 (2024). - J. Schott, R. A. Berner, X-ray photoelectron studies of the mechanism of iron silicate dissolution during weathering. Geochim. Cosmochim. Acta 47, 2233–2240 (1983).
- M. Van Den Berghe, N. Merino, K. H. Nealson, A. J. West, Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology, 13 (2021).
- A. Lunstrum, M. Van Den Berghe, X. Bian, S. John, K. Nealson, A. J. West, Bacterial use of siderophores increases olivine dissolution rates by nearly an order of magnitude. Geochem. Perspect. Lett. 25, 51–55 (2023).
- M. Van Den Berghe, N. G. Walworth, N. C. Dalvie,
C. L. Dupont, M. Springer, M. G. Andrews, S. J. Romaniello, D. A. Hutchins, F. Montserrat, P. A. Silver, K. H. Nealson, Microbial Catalysis for CO2 Sequestration: A Geobiological Approach. Cold Spring Harb. Perspect. Biol., a041673 (2023). - R. Gerrits, R. Pokharel, R. Breitenbach, J. Radnik, I. Feldmann, J. A. Schuessler, F. von Blanckenburg, A. A. Gorbushina, J. Schott, How the rock-inhabiting fungus K. petricola A95 enhances olivine dissolution through attachment. Geochim. Cosmochim. Acta 282, 76–97 (2020).
- R. Pokharel, R. Gerrits, J. A. Schuessler, F. von Blanckenburg, Mechanisms of olivine dissolution by rock-inhabiting fungi explored using magnesium stable isotopes. Chem. Geol. 525, 18–27 (2019).
- R. R. Singhania, A. K. Patel, A. Pandey, “The Industrial Production of Enzymes” in Industrial Biotechnology, W. Soetaert, E. J. Vandamme, Eds. (Wiley, ed. 1, 2010; https://onlinelibrary.wiley.com/doi/10.1002/9783527630233.ch5), pp. 207–225.
- G. M. Gadd, Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156, 609–643 (2010).
- M. Van Den Berghe, Solving the carbon storage gap with ocean-based enhanced weathering reactor technologies. Open Access Gov., 418–419 (2025).


