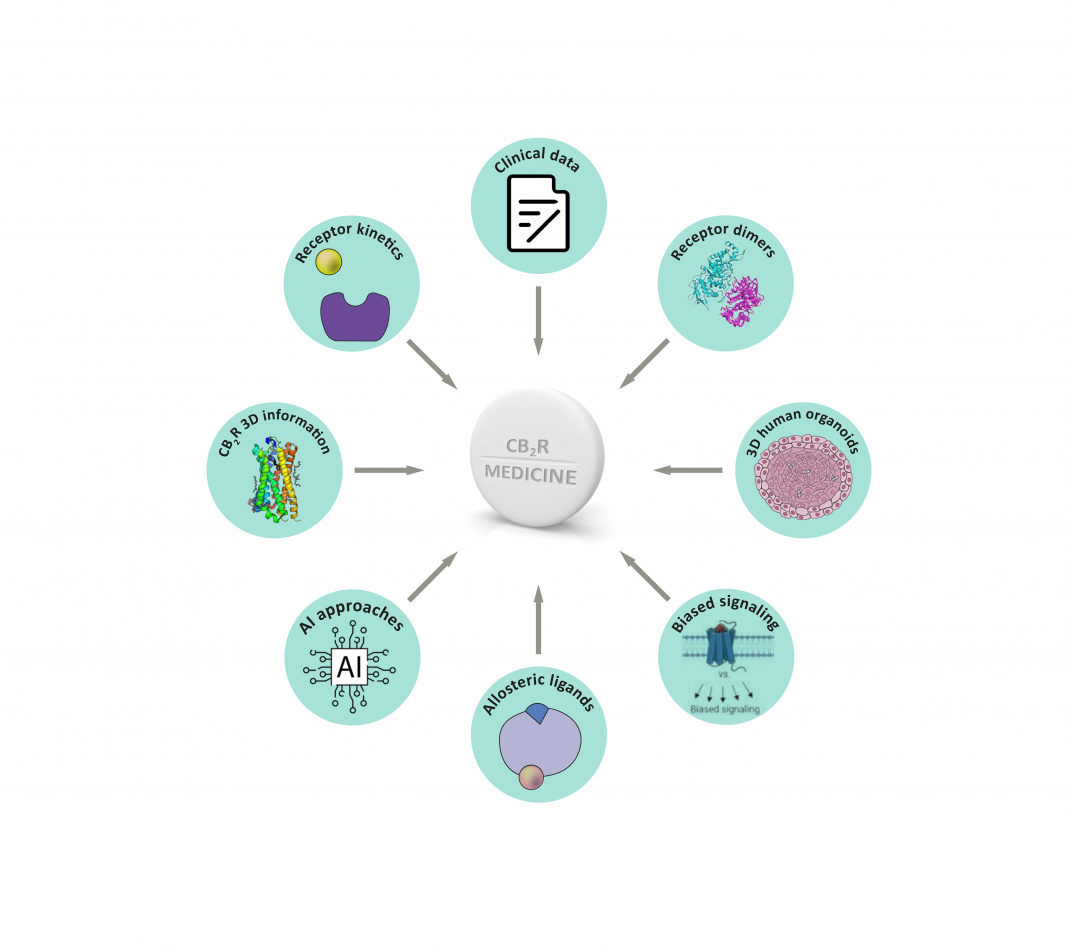
Researchers discuss how scientific innovations might influence the discovery of future tailor-made CB2R-based anti-inflammatory treatments
The G-protein-coupled receptor (GPCR) type-2 cannabinoid receptor (CB2R) is a 2 highly promising pharmacological target for novel anti-inflammatory medicines. (1) CB2R is an essential component of a vital lipid signaling system that tremendously influences multiple human health and disease states ranging from metabolic disorders to neurodegenerative diseases and is termed endocannabinoid system (ECS). Few CB2R-activation-based drugs have been launched. (2)
Several candidates are under active clinical development, aiming at treating diseases in which inflammatory processes play a significant role or are the underlying reason. Recent scientific innovations in ECS research and drug discovery in general (Figure 1) are now being incorporated into the design, synthesis and evaluation of novel ligands targeting CB2R. They will impact the generation of further improved tailor-made medicines for inflammatory diseases.
From homology models to 3D structures of CB2R
Only recently, there was more understanding of how CB2R ligands interact with its target protein due to the lack of reliable information about the 3D structure of the CB2R. The design of novel molecules depended on site-directed mutagenesis data or homology models based on the crystal structure of the somewhat distantly related β2-adrenergic receptor.
Recent elucidation of CB2 receptor structures bound to agonists and inverse agonists by x-ray crystallography and cryogenic electron microscopy provided deep insights into the multiple essential protein-ligand interactions and underlying receptor dynamics of the activation process. (3) This information allows for rationalising structure-function relationships and will tremendously impact and accelerate the design of future ligands.
Novel artificial intelligence approaches for drug discovery
Computational methods support the discovery of CB2 ligands since several years. Novel machine learning methods, e.g., quantitative structure-activity relationship models and de novo molecular design, led to numerous valuable applications in drug discovery. (4)
Recently introduced techniques such as geometric deep learning or de novo drug design applying chemical language models offer further opportunities to accelerate drug design and discovery.
These innovative artificial intelligence-based approaches enable researchers to conceive and select better molecules faster. Thus, the number of necessary design-make-test cycles to arrive at optimal molecules will significantly reduce.
Better understanding of receptor pharmacology
While recently developed high-quality labelled CB2R fluorescent and PET probes have significantly contributed to a better understanding of CB2R expression and function , numerous unanswered questions remain to be addressed regarding the receptor’s mechanism of action and how this translates to a clinical benefit.
For example, the biased signaling induced by different ligands elicited by the existence of different ligand-dependent CB2R conformations is yet poorly understood but highly relevant as it results in distinct pharmacological responses (6). Furthermore, determining binding kinetics, i.e., residence time of a ligand, may provide essential insights and rationalisation for in vivo efficacy and might even influence signaling bias. (7)
Alternatively, allosteric ligands that bind to a site that is topographically distinct from the orthosteric site of the endogenous ligand might provide a superior therapeutic solution as they can modulate affinity and/or efficacy of endogenous ligands.
Lastly, balancing the association equilibria of CB2R receptors homo and heterodimers might translate into different functional properties and ultimately address different receptor populations for arriving at tailor-made CB2R therapeutics.
Incorporation of translational knowledge
Development candidates often fail in phase 2 clinical trials where the focus is on establishing a preliminary efficacy of the drug in patients, usually against a placebo control group [2b]. The main reason is the lack of translatability from promising preclinical in vivo efficacy studies toward the human situation.
Thus, a feedback loop and back-translation of clinical data and knowledge from patients or human ‘systems’ toward preclinical research will tremendously increase the chance of being successful. Therefore, steady feeding of discovery activities with the most recent clinical data and information on biomarkers are instrumental.
Furthermore, bioinformatics approaches that help bridge between species can contribute to clinical success. The recently introduced use of human stem cell-derived three-dimensional multi-cellular microtissues that closely mimic the complex structure and functionality of human organs, so-called organoids, became increasingly important for drug discovery. They allow, e.g., drug screens and toxicity evaluations in human systems.
Overall, the recently elucidated 3D structures of CB2R, novel artificial intelligence approaches for drug discovery, an improved understanding of receptor pharmacology, and last but not least, the incorporation of the accumulated translational knowledge will enable the discovery of more potent, effective and safe CB2R medicines for indications with a dire or even unmet medical need, eventually leading us to much more significant benefits for patients in the treatment of inflammatory diseases.
References
-
- a) Maccarrone M, Di Marzo V, Gertsch J et al. (2023) Goods and bads of endocannabinoid system as a therapeutic target: Lessons learned after 30 years. Pharmacol Rev. Epub ahead of print, doi: 10.1124/pharmrev.122.000600;
- b) Maccarrone M, Grether U (2022) Is CB2R a hidden treasure trove for treating inflammatory diseases? Open Access Government, https://www.openaccessgovernment.org/ article/cb%e2%82%82r-treating-inflammatory-diseases- endocannabinoid-system/145866/
-
- a) Brennecke B, Gazzi T, Atz K et al. (2021) Cannabinoid receptor type 2 ligands: an analysis of granted patents since 2010. Pharm Pat Anal 10:111-163;
- b) Grether U, Nazaré M (2023) CB2R agonists in the clinics: A treasure chest for treating inflammatory diseases. Open Access Government, https://www.openaccessgovernment.org/ article/cb2r-agonists-clinics-treating-inflammatory- diseases/163225/
-
- a) Li X, Shen L, Hua T, Liu ZJ (2020) Structural and Functional Insights into Cannabinoid Receptors. Trends Pharmacol Sci. 41(9):665-677;
- b) Guba W, Nazaré M, Grether U (2020) Natural Compounds and Synthetic Drugs to Target Type-2 Cannabinoid (CB2) Receptor, in New tools to interrogate endocannabinoid signaling. From natural compounds to synthetic drugs (Maccarrone M ed) pp 89-167, Royal Society of Chemistry, Cambridge.
- Atz K, Guba W, Grether U, Schneider G. (2023) Machine Learning and Computational Chemistry for the Endocannabinoid System. Methods Mol Biol. 2576:477-493.
- Nazaré M, Grether U (2023) Visualizing the anti-inflammatory cannabinoid Type-2 receptor. Open Access Government, https://www.openaccessgovernment.org/ article/visualizing-the-anti-inflammatory-cannabinoid- type-2-receptor/155448/
- Morales P, Goya P, Jagerovic N (2018) Emerging strategies targeting CB2 cannabinoid receptor: Biased agonism and allosterism. Biochem Pharmacol. 157:8-17.
- Martella A, Sijben H, Rufer AC et al (2017) A Novel Selective Inverse Agonist of the CB2 Receptor as a Radiolabeled Tool Compound for Kinetic Binding Studies. Mol Pharmacol. 92(4):389-400.
Acknowledgements
We want to thank all colleagues who have contributed to our investigations on CB2R over the years.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.