Expert scientists working on endocannabinoid system (ECS) trials explain how CB₂R can be used to treat inflammatory diseases
Receptors are important protein structures that allow cells to interact with their environment or control activities within a cell. The largest group of receptors are G-protein-coupled receptors (GPCRs) and encompasses eight hundred members encoded by approximately 3% of the human genome. GPCRs play an important role in multiple physio-pathological conditions throughout the body, including treating inflammatory diseases [1].
Type-2 cannabinoid receptor – A key element of the endocannabinoid system
Type-2 cannabinoid receptor (CB₂R) [2] is a highly important GPCR and represents a promising target for developing novel therapeutics to treat a plethora of diseases linked to its deregulation. CB₂R and the closely related type-1 cannabinoid receptor (CB₁R) [3] are essential elements of the endocannabinoid system (ECS). The ECS plays a key role for human health and disease state and consists of: i) endocannabinoids (eCBs), a class of endogenous CB½R ligands, whose main representative members are N-arachidonoylethanolamine (anandamide) and 2-arachidonylglycerol (2-AG); ii) receptor targets other than CB₁R and CB₂R that can be bound and activated by eCBs, along with their interacting proteins; and iii) metabolic enzymes and transporters involved in the control of eCB lifespan, and hence biological activity [4].
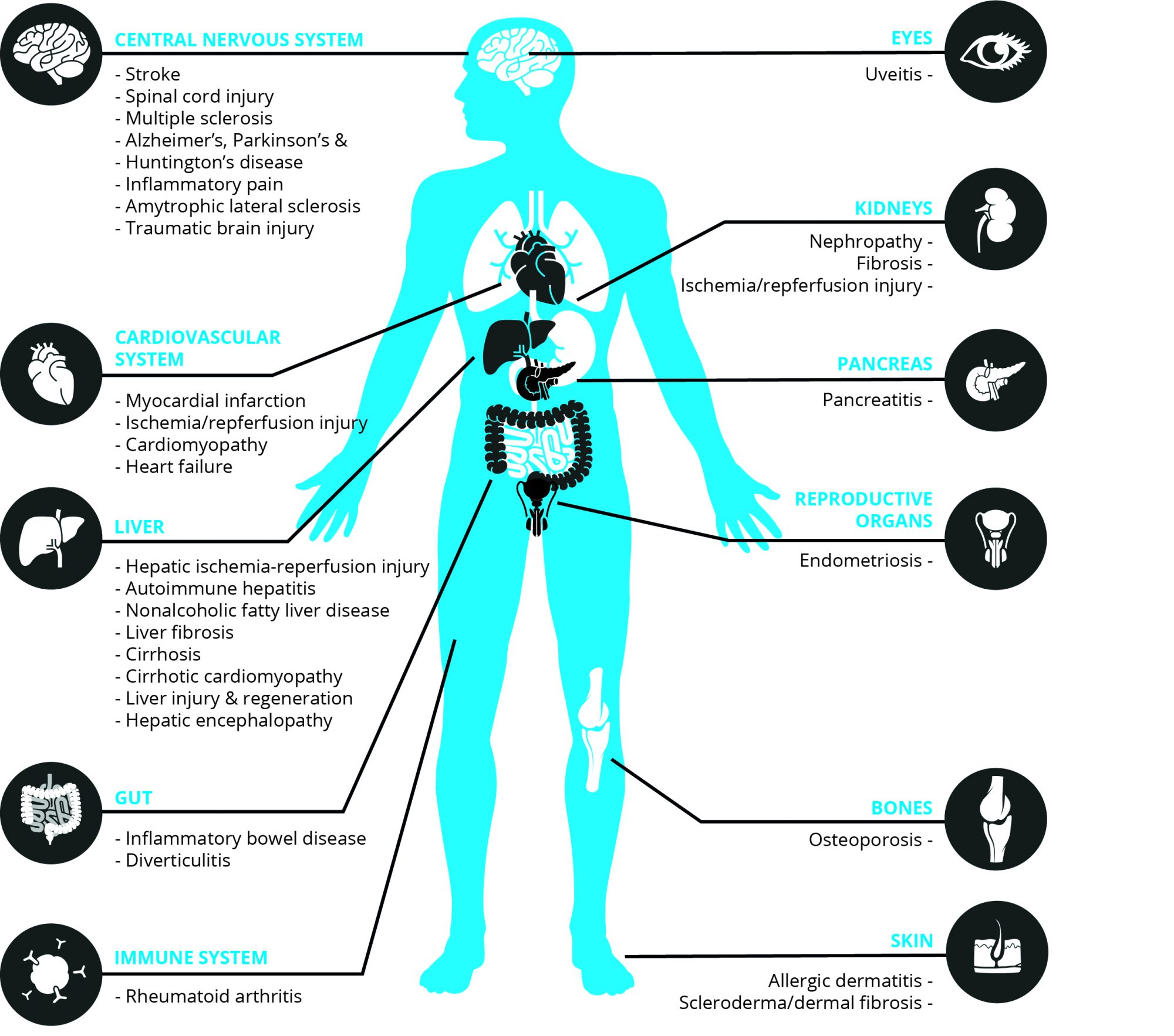
Where is CB₂R located?
While CB₁R is mainly found in the central nervous system (CNS), CB₂ receptors are primarily expressed in all tissues and circulating cells of the immune system. Furthermore, moderate to low CB₂R expression has been reported in other peripheral districts (e.g. hepatic myofibroblasts, cardiomyocytes, endothelial smooth muscle cells) [5] and in the CNS (e.g. microglial cells and retinal cells) [6]. The degree of CB₂R expression and activity depends on the type and activation of cells, as well as on the stimulus. Under various pathological conditions/disease states CB₂R expression can be markedly unregulated in affected tissues/cells [7]. This is often accompanied by elevated eCB levels as a part of the inflammatory response/tissue injury [7].
How can CB₂R activation treat inflammatory diseases? Increased local eCB levels and CB₂R expression upon inflammation/tissue injury trigger fast signaling responses in immune and other cells leading mostly to a sequence of activities of a protective nature. Furthermore, eCBs and/or synthetic CB₂R activators have been reported to attenuate inflammation and associated tissue injury in a huge number of pathological conditions/ diseases, ranging from cardiovascular, gastrointestinal, liver, kidney, lung, neurodegenerative/neuroinflammatory, skin pathologies, rheumatoid arthritis, endometriosis, to eye diseases [7,8] (Figure 1).
Ligands targeting CB₂R
Due to its enormous therapeutic potential many academic and industry institutions came up with novel ligands targeting CB₂R. First publications and patents appeared in 1991 [9] and 1996 [10], respectively. Since then, more than 750 CB₂R patent applications have been filed, the majority of them describing activators of the receptor. CB₂R molecules can be subdivided into eCBs, plant-derived cannabinoids (phytocannabinoids), cannabinoid-like and synthetic CB₂R ligands. In recent years research efforts concentrated on generating small molecules that are either highly selective against CB₁R or possess limited blood–brain barrier (BBB) permeability, to avoid the psychotropic effects resulting from CB₁R activation in the brain [11], such as for the dual CB₁R/CB₂R agonist Δ⁹-tetrahydro-cannabinol (Δ⁹-THC), the active ingredient of Cannabis sativa [12].
CB₂R ligands in clinical trials
Overall more than 20 activators of CB₂R have been or are currently being studied in humans [13]. They cover a broad range of indications encompassing neurological and fibrotic diseases, pain, osteoarthritis and cancer. Three phytocannabinoid-derived dual CB₁R/CB₂R agonists have been launched. Currently, more than 7 CB₂R ligands are under active clinical 2 development. In contrast to first clinically evaluated CB₂R agonists, which often were also activating CB₁R and were designed for pain indications, recent focus shifted toward peripheral indications with an inflammatory and/or fibrotic background.
Future perspectives on the understanding of CB₂R Significant progress in understanding CB₂R expression, mechanism of action and translatability of results toward the human situation has been made in the past years. Furthermore, a multitude of high quality ligands targeting CB₂R have been generated. Applying this knowledge in the right context and condition for attenuating diseases with an inflammatory/tissue injury background holds great promise to guide us to unlock the receptor’s full therapeutic potential, ultimately leading to patient benefit.
References
[1] Thomsen, W.; Frazer, J.; Unett, D. Functional Assays for Screening GPCR Targets. Curr Opin Biotechnol 2005, 16 (6), 655–665. https://doi.org/10.1016/j.copbio.2005.10.008.
[2] a) Munro, S.; Thomas, K. L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365 (6441), 61–65. https://doi.org/10.1038/365061a0; b) Griffin, G.; Tao, Q.; Abood, M. E. Cloning and Pharmacological Characterization of the Rat CB(2) Cannabinoid Receptor. J Pharmacol Exp Ther 2000, 292 (3), 886–894.
[3] Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 34, 605–613 (1988).
[4] a) Maccarrone, M. Missing Pieces to the Endocannabinoid Puzzle. Trends Mol Med 2020, 26 (3), 263–272. https://doi.org/10.1016/j.molmed.2019.11.002; b) Guba, W.; Nazaré, M.; Grether, U. Chapter 4. Natural Compounds and Synthetic Drugs to Target Type-2 Cannabinoid (CB2) Receptor. In RSC Drug Discovery Series; 2020; Vol. 2021- Janua, pp 89–167. https://doi.org/10.1039/9781839160752-00089.
[5] a) Weis, F.; Beiras-Fernandez, A.; Sodian, R.; Kaczmarek, I.; Reichart, B.; Beiras, A.; Schelling, G.; Kreth, S. Substantially Altered Expression Pattern of Cannabinoid Receptor 2 and Activated Endocannabinoid System in Patients with Severe Heart Failure. J Mol Cell Cardiol 2010, 48 (6), 1187–1193. https://doi.org/10.1016/j.yjmcc.2009.10.025; b) Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Huffman, J. W.; Mackie, K.; Pacher, P. CB 2 Cannabinoid Receptor Agonists Attenuate TNF- α-Induced Human Vascular Smooth Muscle Cell Proliferation and Migration. Br J Pharmacol 2008, 153 (2), 347–357.https://doi.org/10.1038/sj.bjp.0707569.
[6] a) Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurode- generative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front Neurosci 2017, 11, 30. https://doi.org/10.3389/fnins.2017.00030; b) Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast 2016, 2016, 1–10. https://doi.org/10.1155/2016/2916732.
[7] a) Viscomi, M.T.; Oddi, S.; Latini, L.; Pasquariello, N.; Floren- zano, F.; Bernardi, G.; Molinari, M.; Maccarrone, M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci 2009, 29(14):4564-70. https://doi.org/10.1523/JNEUROSCI.0786-09.2009; b) Pacher, P.; Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011, 50(2), 193–211.
[8] Gasperi, V.; Guzzo, T.; Topai, A.; Gambacorta, N.; Ciriaco, F.; Nicolotti, O.; Maccarrone, M. Recent Advances on Type-2 Cannabinoid (CB2) Receptor Agonists and Their Therapeutic Potential. Curr. Med. Chem. 2022, in press. https://doi.org/10.2174/0929867329666220825161603.
[9] Bell MR, D’Ambra TE, Kumar V et al. Antinociceptive (aminoalkyl)indoles. J. Med. Chem. 34(3), 1099–1110 (1991).
[10] Sanofi SA.: FR2735774A1 (1996).
[11] a) Marsicano G, Lutz B. Neuromodulatory functions of the endocannabinoid system. J. Endocrinol. Investig. 29(Suppl.3), 27–46 (2006); b) Kano M, Ohno-Shosaku T, Hashimoto-dani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89(1), 309–380 (2009).
[12] a) Devane, W. A.; Dysarz, F. A.; Johnson, M. R.; Melvin, L. S.; Howlett, A. C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol Pharmacol 1988, 34 (5), 605–613; b) Matsuda, L. A.; Lolait, S. J.; Brownstein, M. J.; Young, A. C.; Bonner, T. I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346 (6284), 561–564. https://doi.org/10.1038/346561a0.
[13] Benjamin Brennecke, Thais Gazzi, Kenneth Atz, Jürgen Fingerle, Pascal Kuner, Torsten Schindler, Guy de Weck, Marc Nazaré, Uwe Grether; CB2 Receptor Ligands – An Analysis of Granted Patents since 2010. Pharm. Pat. Anal. (2021), epub ahead of print, https://www.future-science.com/doi/10.4155/ppa-2021-0002.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


