In this article, Dr Alan Herbert discusses how different types of cellular scaffolds interact and impact the risk of diseases, citing the example of Z-RNAs pushing cells to inflammatory states in tumors and autoimmune conditions, setting the stage for new therapeutics
The cell is full of so many proteins and RNA versions that it is a challenge to see how anything works at all. There is so much crowding it is like trying to get to your train through the mass of people on the station platform during rush hour. All the different biomolecules involved can be modified by the addition of phosphates, sugars, and lipids in so many different ways and at so many alternative sites that it seems impossible to track the one that matters. Yet, somehow, cells work in a highly efficient manner.
Cellular scaffolding
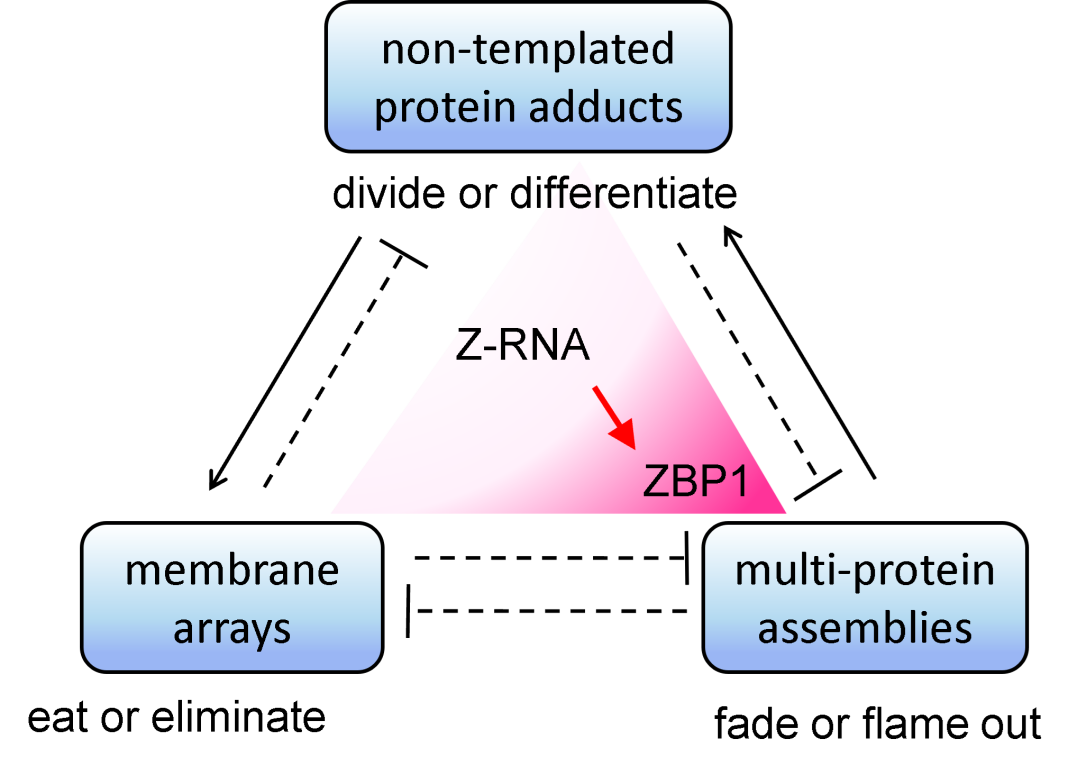
While the participants in this molecular chaos are genetically encoded, the outcomes are not. Rather, the cell is just interested in assembling the scaffolds most responsive to its ever-changing state. On these frameworks is layered the patchwork of proteins that decide a cell’s fate. In that crowded cytoplasm, there are three major classes of non-genetically templated scaffolds found. One framework involves the decoration of proteins with polymers made from sugars and nucleotides, and even through the repeated addition of another protein.
The latter outcome is exemplified by the chains of ubiquitin that are attached to proteins reiteratively. These covalently bonded adducts can be quite long and anchored through different ubiquitin amino acids, creating both linear and tree-like polymers. The ubiquitin addons nucleate a series of proteins that drive the response. They assemble what is called a signalosome. Usually, these effectors drive the proliferation and differentiation of cells at an early stage in their development.
Another non-genetically templated scaffold is based on membrane arrays. They provide a two-dimensional surface onto which proteins can assemble. Their formation is controlled by the cell’s metabolic state. For example, in well-fed cells, the well-known mTOR complex forms on lysosomal membranes to turn off the lysosomal system that starved cells depend upon. Under these metabolic conditions, there is no need to recycle cellular components in order to survive. The membrane system can also capture or engulf other scaffolds, like the signalosome, to terminate their action.
A third scaffold is formed from proteins that bind to each other through short peptide motifs. The assembly provides a platform to activate downstream effector pathways. Such scaffolds include those that promote the formation of inflammatory protein cascades called inflammasomes. A different protein framework regulates cell death. These pathways silently remove surplus cells or those that have passed their expiry date. Alternatively, they stroke inflammatory cell death responses to activate antiviral or anti-tumor responses to eliminate a threat.
The state of the cell depends on balancing the activities of these three types of scaffolds. In normal cells, the activation of one scaffold is held in check by the other two. For example, the membrane system can remove signalosomes. It also destroys the inflammatory protein platforms. As a consequence of these interactions, scaffolds are in a state of flux. They grow and shrink, capturing and concentrating components from their surroundings, falling apart when parts are missing. At some critical mass, they form a distinct structure that separates the active complex from the rest of the cellular crowd. The ensemble then helps promote the effector response.
An example of how these scaffolds is genetically mapped is given in the recent paper in Genes and Immunity from Dr Herbert at InsideOutBio. He describes how the platforms are modified by the environment to produce non-genetically encoded outcomes. The paper focuses on osteoporosis as the various pathologies can be tracked because the cellular players involved remain trapped within the bone, giving insight into their roles.
How cellular scaffolding informs disease
Disease is more likely when the activity of one type of scaffold dominates those of the others. For example, signalosomes drive the proliferation of cancer cells. Futile cycles that prevent a cell from developing further arise when membranes engulf and eliminate cellular components as soon as they are formed. Further, unrestrained formation of protein scaffolds can promote chronic inflammatory diseases. Likewise, the loss of components essential to form a particular scaffold type can also produce disease outcomes. For example, failure of signalosomes to assemble will lead to loss of a tissue’s ability to replenish itself, impairment of the membrane scaffold formation can lead to the accumulation of protein aggregates that are toxic to a cell as seen in Alzheimer’s Disease, while loss of protective protein-platforms essential for host immunity predisposes to chronic infection.
Z-RNA in scaffold formation
The cell can regulate the formation of these scaffolds in many ways. Noncoding RNAs can play an important role. In recent work, the key part played by the unusual left-handed Z-RNA helix in scaffold formation has come to light.
The Z-RNAs are encoded by sequences in the genome called flipons. The sequences fold initially to form double-stranded RNA (dsRNA), adopting the right-handed double-helix conformation called A-RNA. The flip to the left-handed Z-RNA n localizes enzymes to edit the dsRNA and mark these RNAs for destruction, preventing their accumulation.
At first sight, this process seems wasteful. Why make dsRNA only to destroy it? It turns out that many of the dsRNAs derive from retroelements that arose by copying and pasting themselves into the genome, a process that depends on Z-RNA or Z-DNA formation (we currently don’t know which). They cause damage by inserting into active genes. The Z-RNA-mediated processes that destroyed these dsRNAs helped stop their spread. However, over time, the Z-RNAs formed by these retroelements in host transcripts came to tag the RNAs as host-derived, allowing the host to discriminate self-RNAs from those of viruses and other pathogens that infiltrate cells.
The interferon response to these invaders induces host responses that lead to the expression of additional Z-RNA forming flipon transcripts that overwhelm the ability of cells to terminate the response. In addition, interferon greatly increases the expression of ZBP1, a protein that is triggered by Z-RNA to activate inflammatory cell death (see scarlet coloring in the figure). The response eliminates infected cells. It also kills dysfunctional cells that highly express these same Z-RNA-forming transcripts due to faulty gene readout. Such a process occurs in cancer cells or when aging cells persist rather than fading out gracefully. Accumulation of Z-RNAs in stress granules also occurs when cells are unable to efficiently translate RNA into protein. Such processes occur in chronic neurodegenerative diseases like Alzheimer’s and in autoimmune states like systemic lupus erythematosus. These new findings have spurned the development of novel flipon therapeutics to target the Z-DNA and Z-RNA pathways.
Overall, the crowded cell is in constant churn. Scaffolds are seeded as a response to ongoing events. They differ in how quickly they form, how large they become, and how they interact with each other. Often, the scaffolds are layered, with one coat seeding the next. The stepwise construction sources components from the cellular milieu, with checks and balances at each stage. Many of the proteins individually have a small effect on the overall response. The components are genetically encoded, but the outcomes are not. The response depends on the wisdom of the crowd. Some crowds perform better than others.
References
- Herbert A. Osteogenesis imperfecta type 10 and the cellular scaffolds underlying common immunological diseases. Genes Immun. 2024. Epub 20240529.
doi: https://doi.org/10.1038/s41435-024-00277-4. PubMed PMID: 38811682. - Herbert A. Flipons and the logic of soft-wired genomes. 1st ed. Boca Raton: CRC Press; 2024. https://tinyurl.com/2rn53mzx (PDF); https://tinyurl.com/yar676se (eBook)

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.