Drug hunters explain how to overcome pitfalls on the way to CB2R medicine and therapeutics
Type-2 cannabinoid receptor (CB2R) is a key element of the endocannabinoid system (ECS) and an important pharmacological target which was referred to as a ‘hidden treasure trove for treating inflammatory diseases’ [1]. Preclinical data suggest that activation of this G-protein-coupled receptor (GPCR) holds great potential for treating a plethora of pathological conditions in humans e.g. cardiovascular, gastrointestinal, liver, kidney, lung, neurodegenerative/ neuroinflammatory, skin pathologies, rheumatoid arthritis, endometriosis, and eye diseases [2]. Despite this enormous therapeutic potential, no selective CB2R agonist has reached the market so far as CB2R medicine.
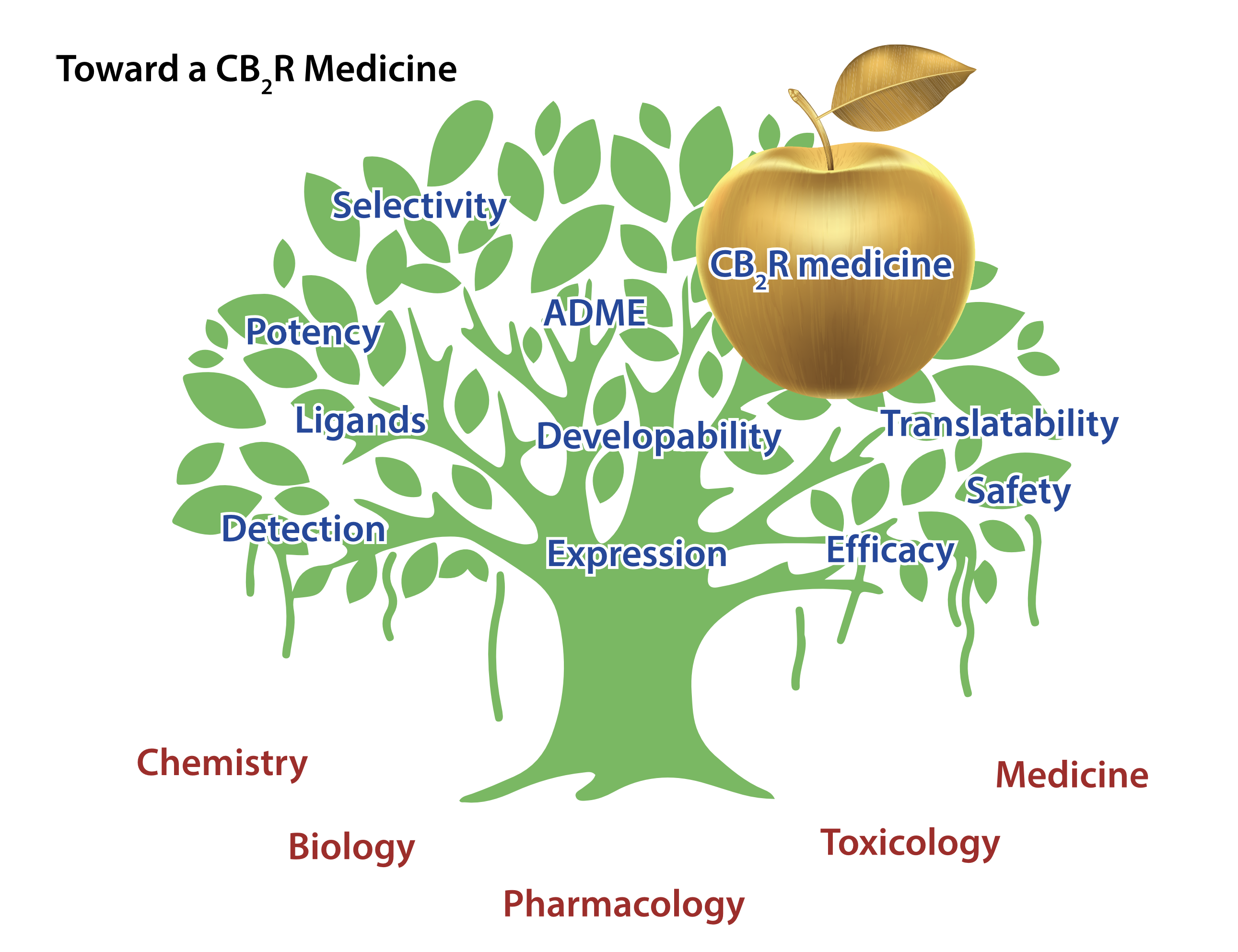
This can be linked to four main reasons:
- Expression & detection of CB2R;
- Quality of early tool compounds;
- Profile of first development candidates;
- Translatability of preclinical efficacy data toward humans.
All these elements are highly relevant for any drug discovery program but proved to be particularly challenging for CB2R (Figure 1).
How to detect CB2R?
The detection of CB2R relies on assays of messenger RNA (mRNA) expression or methodologies which can detect the protein itself. Meanwhile, sophisticated mRNA techniques such as quantitative real-time polymerase chain reaction (qRT-PCR), droplet digital PCR (ddPCR) and RNAscope have revealed that CB2R is under healthy conditions primarily expressed in immune cells and detailed information has been reported in multiple databases such as Genotype-Tissue Expression (GTEx) and the ImmGen Web Page [3].
Various states of a disease can lead to robust induction of CB2R. Due to a paucity of specific antibodies, the detection of CB2R protein remained for a long time challenging. Only recently highly selective labelled chemical probes such as radiotracers and fluorescent ligands have been developed that allow for more reliable detection of CB2R protein [4].
The importance of high-quality chemical tools for studying CB2R pharmacology
Target validation is an essential element of drug discovery and requires tool compounds. Such chemical tools need to fulfil a multitude of criteria, e.g., high potency on the target and excellent selectivity regarding off-targets. If applied in vivo, tool compounds need to be bioavailable meaning they must be able to reach the target protein within the relevant diseased tissue compartment in sufficient concentration to trigger a pharmacological effect.
For extrapolation toward the human situation potency and selectivity profiles of chemical probes must be comparable in the species in which an in vivo efficacy study is conducted and in humans. Initial CB2R probes were lacking one or 2 more of the above-mentioned criteria leading to misinterpretations of in vivo efficacy data. To address this issue, an extensive molecular pharmacology characterization of the most widely used CB2R ligands in a collaborative effort between multiple academic and industry laboratories was conducted, reaching a consensus on which CB2R molecules to use for studying the role of CB2R in biological and diseases processes [5].
Profile of early development candidates
Due to the huge therapeutic potential of CB2R multiple ligands have been developed by both academic institutions and the pharmaceutical industry [6]. These ligands can be divided into endogenous cannabinoids and related fatty acid derivatives, phytocannabinoids and synthetic cannabinoids. Besides the abovementioned criteria for chemical tools, drug candidates need to fulfil a multitude of further requirements, e.g., regarding their ADME, safety and developability properties. Early development candidates often suffered, e.g., from high lipophilicity and poor solubility, contributing to unfavorably low oral bioavailability. Furthermore, their therapeutic index was limited due to insufficient selectivity against CB1R. Meanwhile, more than 20 new molecular entities activating CB2R have been evaluated in clinical trials and in particular, most recent ligands have overcome deficiencies of the early ligands, possess excellent ADME properties translating into favourable pharmacokinetic profiles and are devoid of centrally mediated CB1R-driven psychotropic or other toxicological side effects.
Translatability of preclinical efficacy data toward human situation
Insufficient selectivity against CB1R could partially explain why some of the early clinical trials with CB2R agonists failed. At high concentrations, such ligands also trigger CB1R activation, which counteracts beneficial CB2R effects in multiple pathological conditions or lead to cardiovascular and other side effects [7].
Furthermore, past clinical trials often looked at the wrong illnesses such as osteoarthritis and third molar extraction, which didn’t match with what preclinical results suggested to be a good patient population. Recent studies put a strong focus on peripheral indications with an inflammatory/immunomodulatory and/or fibrotic background [6] for which there is ample proof of concept in rodent disease models.
Although some uncertainties still surround CB2R and CB2R medicine, researchers made significant progress and are now better armed to solve them. High-quality biological and labelled chemical probes will facilitate a better understanding of CB2R expression and mechanism of action. Chemical tools with suitable property profiles for interrogating CB2R, pharmacology and drug candidates with ideal target compound profiles have been developed to aid preclinical research. Ongoing clinical trials mimic disease conditions which are matching preclinical proof of concept studies and thus hold great potential for unlocking CB2R’s promise for the treatment of inflammatory diseases.
References
- a) Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61-65; b) Griffin G, Tao Q, Abood ME (2000) Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 292: 886-894; c) Maccarrone M, Grether U (2022) Is CB2R a hidden treasure trove for treating inflammatory diseases? Open Access Government, https://www.openaccessgovernment.org/article/cb%e2%82%82r-treating-inflammatory-diseases-endocannabinoid-system/145866/.
- a) Pacher P, Mechoulam R (2011) Is lipid signalling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50: 193-211; b) Gasperi V, Guzzo T, Topai A et al. (2022) Recent Advances on Type-2 Cannabinoid (CB2) Receptor Agonists and Their Therapeutic Potential. Curr Med Chem https://www.doi.org/10.2174/0929867329666220825161603.
- a) GTExPortal, https://gtexportal.org/, (accessed November 2022); b) ImmGen Web Page, http://www.immgen.org/, (accessed October 2022).
- a) Haider A, Gobbi L, Kretz J et al. (2020) Identification and Preclinical Development of a 2,5,6-Trisubstituted Fluorinated Pyridine Derivative as a Radioligand for the Positron Emission Tomography Imaging of Cannabinoid Type 2 Receptors. J Med Chem 63: 10287-10306; b) Sarott RC, Westphal MV, Pfaff P et al. (2020) Development of High-Specificity Fluorescent Probes to Enable Cannabinoid Type 2 Receptor Studies in Living Cells. J Am Chem Soc 142: 16953-16964; c) Gazzi T, Brennecke B, Atz K et al. (2022) Detection of cannabinoid receptor type 2 in native cells and zebrafish with a highly potent, cell- permeable fluorescent probe. Chem Sci 13: 5539-5545.
- Soethoudt M, Grether U, Fingerle J et al. (2017) Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 8: 13958.
- a) Guba W, Nazaré M, Grether U (2020) Natural Compounds and Synthetic Drugs to Target Type-2 Cannabinoid (CB2) Receptor, in New tools to interrogate endocannabinoid signaling. From natural compounds to synthetic drugs (Maccarrone M ed) pp 89-167, Royal Society of Chemistry, Cambridge; b) Brennecke B, Gazzi T, Atz K et al. (2021) Cannabinoid receptor type 2 ligands: an analysis of granted patents since 2010. Pharm Pat Anal 10: 111-163.
- a) Rogers N (2015) Cannabinoid receptor with an ‘identity crisis’ gets a second look. Nat Med 21: 966-967; b) Pacher P, Steffens S, Haskó G et al. (2018) Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 15: 151-166.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


