Charles W Carter Jr, Department of Biochemistry & Biophysics, University of North Carolina at Chapel Hill, explores prebiotic processes from the historical context enabling the emergence of translation
Many vexing problems surround how information first flowed at the origin of life. None is more self-evident than the question, which came first: genes or proteins? Like Zeno’s paradoxes, the question hides an intellectual sleight-of-hand rooted in our limited perception of time. Darwin’s singular accomplishment was to articulate how genetic variation and selection connect different biological species in time.
Both variation and selection arise from an enabling past. They also update that milieu, enabling subsequent evolutionary change. That insight ties the resolution of the paradox explicitly to historical context. De Grasse Tyson answered the underlying question crisply: “The egg was laid by a bird that was not a chicken”.(1) His reasoning applies perforce to molecular evolution.
Biochemical processes that eventually enabled genetic readout did not emerge from a vacuum. Our very existence implies that pre-existing terrestrial chemistry created ancestors of the first protein genes. It must also have promoted information transfer from one biopolymer to the other.
Thus, the earliest genes and proteins very likely began to store and exchange information from one to the other. They coevolved functionally from the outset. I summarize here contemporary research into that historical context.
Coevolution as chemistry
Consider the emergence of proteins and genes as a very slow, very complex chemical reaction pathway. Temperature, solvent polarity and acidity, the presence of non-reactive molecules or surfaces that accelerate or slow reactions down all influence both the speed and final reaction products.
Chemists follow three types of rules when they experimentally validate likely putative reaction intermediates experimentally. First, they construct a putative intermediate in the laboratory. Next, they show that the intermediate forms rapidly enough from the previous intermediate to be on the pathway. Finally, they show that the intermediate itself reacts fast enough to produce the next intermediate.
However, in earlier parts of this series, I’ve described feasible experimental models for examining later stages of the overall process.
In this article, I summarise research areas that are now establishing clearer pictures of the historical context that likely gave rise to the first protein and nucleic acid polymers and how they might have begun to store and exchange information.
Precursors of metabolism
What environmental factors helped different biopolymers coevolve? NASA’s audio-visual programmes(2) provide excellent summaries of their research into early earth environments(3, 4). Synthetic organic chemists have shown how prebiotic chemistry may have produced building blocks necessary to assemble both oligonucleotides and polypeptides(5-8).
Stubbs et al, describe a spontaneous “keto acid cycle”. Intermediate keto acids all are analogues of those in the Krebs tricarboxylic acid cycle at the heart of biological metabolism(9).
Yet, spontaneous rates of succeeding reactions are orders of magnitude faster than those of the Krebs cycle. Two of the seven intermediates – oxaloacetate and α-keto glutarate – are precursors of the amino acids, aspartate and glutamate.
Reaction products themselves often reinforce certain of the pathways that produce them. A key element of proto-metabolic precursors is the idea of autocatalysis – the ability of intermediates themselves to potentiate the chemistry necessary to sustain them(10).
Generating biopolymers
What generated the first proteins and nucleic acids? Joining monomers of both biopolymers requires removing a water molecule. Heat from the sun provides energy without requiring specific molecular energy sources. Repeated cycles of drying and re-wetting drive the assembly of both biopolymers.(3) Tidal motions of the earth’s large bodies of water could have driven dry-wet cycling.
Each biopolymer actually extends the other’s lifetimes, especially if the polypeptides have alternating positively charged sidechains.
That interdependence complements the cyclic nature of the dry-wet cycling. Together, they mark a landmark colonization of the gap between prebiotic synthesis of amino acids and nucleosides and the earliest precursors of genetics.
Molecular recognition and selection
Frenkel-Pinter’s work showed that dry-wet cycling can both assemble the two biopolymers and select those whose structures enable them to bind and stabilize each other. Mutual recognition by oligonucleotides and oligopeptides thus performed a rudimentary selection.
Catalysis, of course, also requires molecular recognition. It is tempting to consider that such recognition might also have created the first biological catalysts(11).
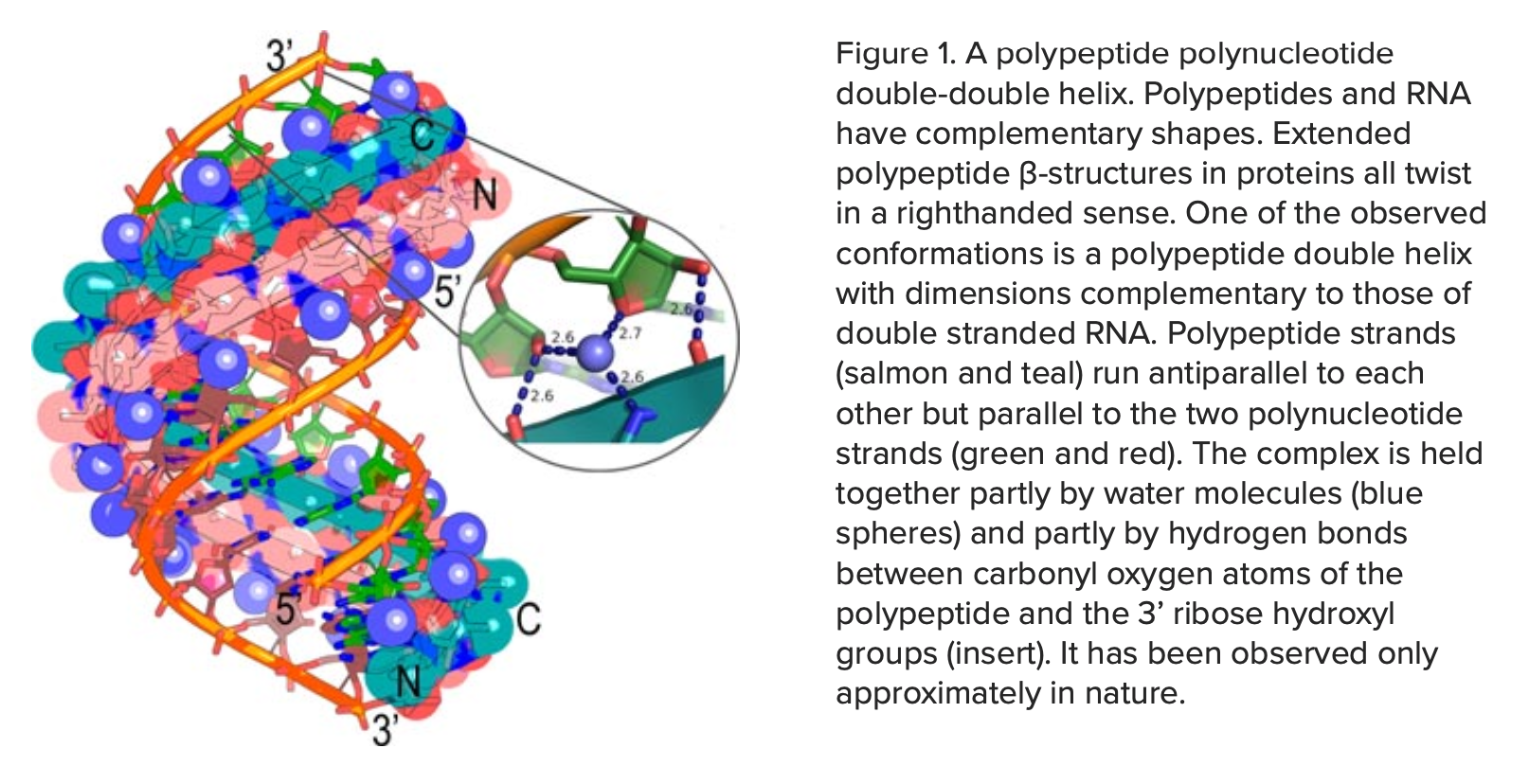
Extended polypeptides can form an antiparallel double helix that is intrinsically complementary to an RNA double helix(12) (Fig. 1). Hydrogen bonds from the peptide to the ribose can form only to a 5’–3’ ribose-phosphate backbone.
Randomly assembled polynucleotides contain frequent 5’–2’ linkages and cannot form such bonds. Moreover, when fitted optimally into the minor groove of an RNA double helix, the stoichiometry is exactly two amino acids per base. This means that bound water molecules and hydrogen bonds between polymers all repeat exactly.
These eerie structural coincidences are necessary and sufficient for heritable sequence information to pass reproducibly between polypeptides and polynucleotides. They may therefore be relevant to the origin of genetics. Such coincidences also abound in physics. Their surfacing also in biology, like the coherence of translated products from opposite strands of the same gene to form the ancestral aminoacyl-tRNA synthetases, pose comparable philosophical challenges.
References
- K. Goldring, WHICH CAME FIRST, THE CHICKEN OR THE EGG?
- Voytek, M., et al., https://www.youtube.com/@pce3prebioticchemistry478/videos. (2020). NASA
- M. Frenkel-Pinter, et. al., NATURE COMMUNICATIONS, 2020, 11, 3137.
- Frenkel-Pinter, M. https://www.youtube.com/watch?v=WyLs12oGqvY(2020). NASA.
- Z. Liu, et. al., Nature Chemistry, 2020, 12, 1023–1028.
- L.-F. Wu and J. D. Sutherland, Emerging Topics in Life Sciences, 2019, 3, 459–468.
- J. D. Sutherland, Angew. Chem. Int. Ed., 2016, 55, 104-121.
- S. A. Benner, et. al., ChemSystemsChem 2019, 1, e1900035.
- R. T. Stubbs, et. al., Nature Chemistry, 2020, 12, 1016–1022.
- S. A. Kauffman, J. Theor. Bio.,, 1986, 119, 1–24.
- K. A. Dill and L. Agozzino, Open Biology, 2020, 11, 200324.
- C. W. Carter, Jr. and J. Kraut, PNAS, USA, 1974, 71, 283-287.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.