Fibrosis was once considered irreversible, resulting from chronic organ damage; Ken-Ichi Kobayashi from Notre Dame Seishin University tells us why the possibility of treatment to reverse the disease is gaining attention
Organ fibrosis is a common phenomenon observed in various organ failure states resulting from disease and aging. The most common examples are renal fibrosis in chronic kidney disease, liver fibrosis in cirrhosis, lung fibrosis in idiopathic pulmonary fibrosis, and pancreatic fibrosis in chronic pancreatitis. The total number of patients with organ fibrosis in Japan is nearly 20 million, and it is no exaggeration to say that it is a ‘national disease.’ Until recently, fibrosis was considered to be the end result of chronic organ damage and an irreversible condition with a poor prognosis.
In recent years, however, fibrosis has begun to be recognized as a reversible process, and the possibility of treatment is gaining attention. It is also becoming clear that fibrosis is caused by complex cellular and inter-organic networks rather than by specific organs, and therefore, common pathological mechanisms are thought to exist even in various types of fibrosis. However, the common pathological mechanism of fibrosis, especially fibrosis-attracting factors, is still an enigma.
Organ fibrosis and quinolinic acid Quinolinic acid is an intermediate metabolite of the kynurenine pathway that biosynthesizes NAD, the active form of niacin, from tryptophan, an essential amino acid. This quinolinic acid is an agonist of N-methyl-D-aspartate (NMDA) receptors – which recognize glutamate and are involved in neuroexcitation – and is known to be neurotoxic. Studies in cranial nerve-related fields have shown that quinolinic acid and other metabolites of the kynurenine pathway are neurotoxic and associated with neurodegenerative disease – the first ‘quinolinic acid hypothesis’ in neurodegenerative diseases. However, the effects of these metabolites on peripheral tissues were unknown.
In this context, quinolinic acid became known to be a protein-bound urinary toxin. Kobayashi et al. hypothesized that quinolinic acid accumulation would also affect renal tissues, so they analyzed the renal function of QPRT knockout mice, which can artificially accumulate quinolinic acid. As a result, they have revealed that quinolinic acid may be an inducer of chronic kidney disease, especially renal fibrosis (the second ‘quinolinic acid hypothesis’ in chronic kidney disease proposed by us).
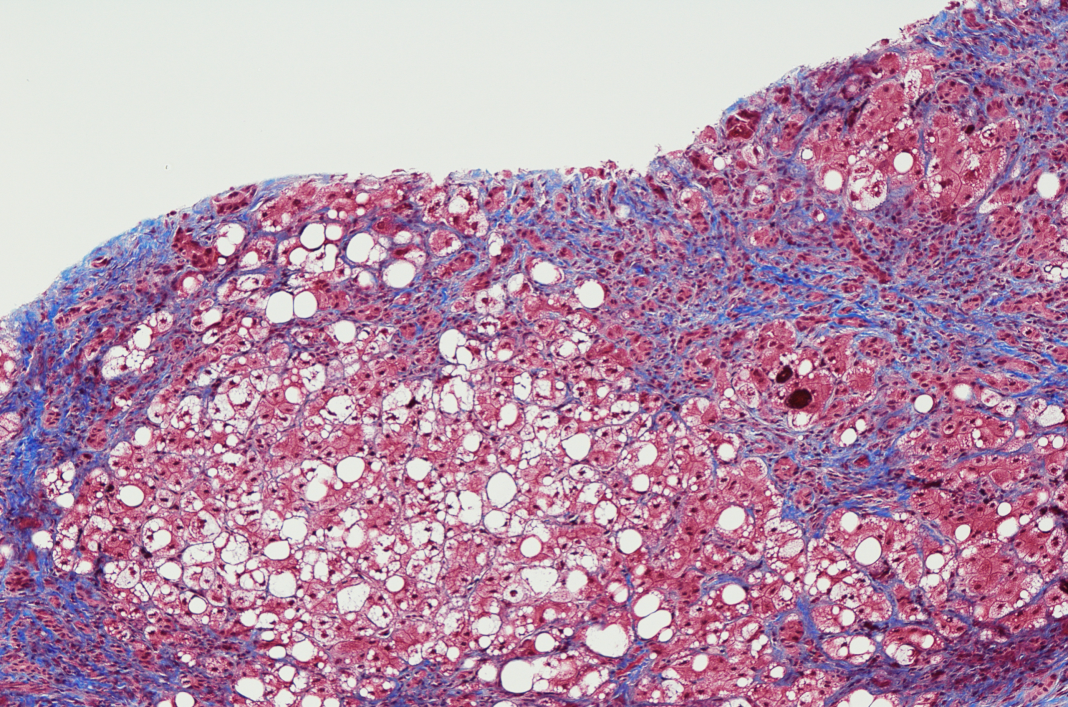
We have also shown that this mouse could be a ‘non-invasive renal fibrosis model,’ which is rare in the world. However, the involvement of quinolinic acid in fibrosis in other organs is unknown. We have used this mouse model to examine the effects of quinolinic acid in the liver, the main quinolinic acid-producing organ in peripheral tissues, and found that quinolinic acid accumulates in stellate cells, the predominant cell type responsible for hepatic fibrogenesis, indicating the possibility that quinolinic acid may affect liver fibrosis.
Based on the above experimental findings, we hypothesized that quinolinic acid is a ‘common’ inducer of organ fibrosis. We are investigating whether or not fibrosis occurs in the liver, pancreas, lung, and heart tissues of QPRT knockout mice.
Liver fibrosis and kynurenine pathway in mouse models of NASH
Non-alcoholic steatohepatitis (NASH) is a metabolic disease associated with liver fibrosis, which often develops against a background of lifestyle-related diseases such as obesity, and the number of patients is rapidly increasing worldwide. Therefore, attention has focused on elucidating the molecular mechanisms underlying the pathogenesis of NASH and on foods that prevent or ameliorate NASH. Recently, Matsumoto et al. (2021) found that brown rice may prevent or suppress NASH by enhancing vitamin A metabolism in the liver.
Although the NAD metabolic pathway has been reported to be affected in the liver of NASH, the effects on NAD metabolism, especially on the kynurenine metabolic pathway, in an ‘obesity-associated’ NASH model are still unknown. Therefore, we focused on the Gubra Amylin NASH (GAN) diet, including high fat (40%), high fructose (22%), and high cholesterol (2%) that can induce NASH with obesity. In NASH model mice, hepatic gene expression of enzymes in the kynurenine metabolic pathway decreased across the board, while gene expression of quinolinic acid catabolic enzyme (QPRT) in the kidney increased, suggesting that quinolinic acid in the body may be increased. The results suggest that quinolinic acid in the body may be increased. We are conducting a detailed analysis of the variation of quinolinic acid in the body of these mice.
Coffee polyphenols inhibit renal fibrosis
Focusing on renal fibrosis, we have investigated the inhibitory effects of coffee polyphenol chlorogenic acid and its degradation products, caffeic acid and quinic acid, on renal fibrosis using a rat kidney interstitial fibroblast cell line (NRK49F) and a human proximal tubular epithelial cell line (HK-2). As a result, we have obtained findings that coffee acid has an inhibitory effect on renal fibrosis, specifically in renal interstitial cells. In the future, we plan to construct a quinolinic acid-induced renal fibrosis model cell and investigate the effect of coffee polyphenols.
Search for functional food ingredients for prevention and improvement of fibrosis using quinolinic acid-induced fibrosis model cultured cells
We have also conducted a QPRT gene knockout fibroblasts cultured cell model using the CRISPR-cas9 system. We have begun to construct quinolinic acid-induced fibrosis model cell cultures. Using these cultured cell lines and QPRT knockout mice, we would like to clarify the functional food ingredients, mainly polyphenols, that have fibrosis-inhibitory effects.
Conclusion
If our research progresses, not only will the pathological mechanism of organ fibrosis be clarified, but also functional foods that protect the body from organ fibrosis based on this mechanism will be created. We are convinced that this will lead to ‘organ strengthening’ and contribute to the extension of healthy life expectancy.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.