Amit Walia, Matthew Shew and Craig A. Buchman from Washington University School of Medicine, detail the role of cognitive function and electrode mapping in cochlear implant performance
In a previous article, we explored various factors influencing cochlear implant (CI) performance, including demographic, audiologic, and surgical variables, as well as the importance of cochlear health, as measured by electrocochleography. Beyond cochlear health, cognitive function, device function and surgical variables all play a role in performance. This section highlights the impact of cognitive function and device mapping to further refine and optimize CI outcomes.
Cognitive function and cochlear health:
Key predictors of cochlear implant performance in noise While most CI users achieve excellent speech perception in quiet environments, many continue to struggle in noisy settings, with at least half experiencing poor performance when background noise is present.
This challenge is particularly pronounced when there is no spatial separation between the speech signal and the noise. Given that most real-life hearing situations involve some level of background noise, poor performance in these conditions can lead to significant communication difficulties and potential safety issues.
For young children, this may result in linguistic developmental delays with associated educational impact. Cognitive function, particularly for tasks requiring auditory processing and attention, is crucial for distinguishing speech from background noise (i.e., squelch). The complexity of real-world listening demands a high level of cognitive engagement, making this an essential area of focus for improving CI outcomes. Recently, the role of cognition has gained attention as a potential explanation for the variability of CI performance in noise.
At Washington University in St. Louis, we sought to better understand the role of cognition in CI performance, particularly in challenging environments that include background noise (e.g., AzBio sentences +10 dB signal-to-noise ratio). (1,2) As we have previously shown, the impact of cochlear health (as determined by electrocochleography) on performance, a key concept in this research is the need to control for cochlear health as we consider the impact of any other variables such as cognition.
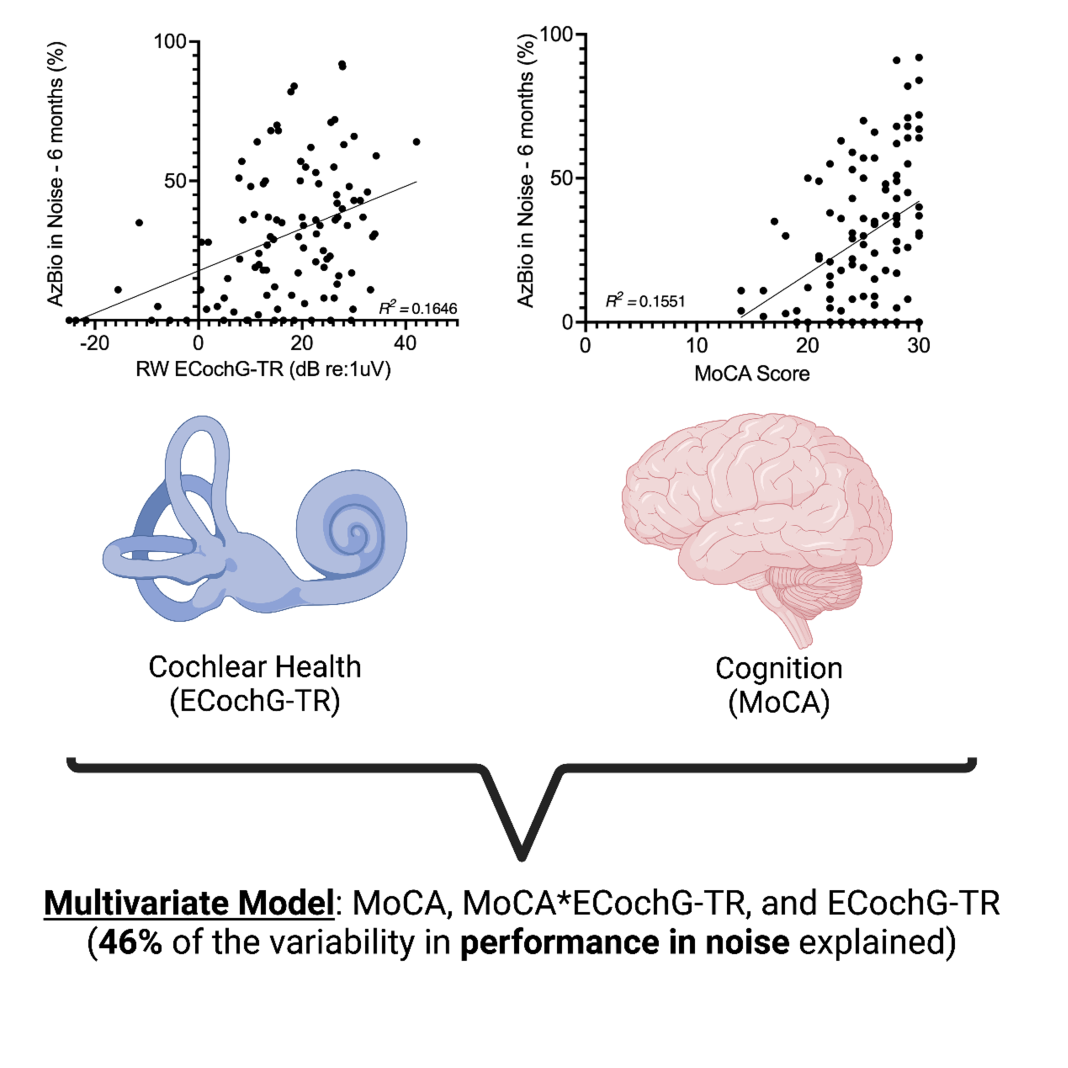
To explore the cognitive aspect of CI performance, we have been routinely collecting Montreal Cognitive Assessment (MoCA) scores for all our patients. The MoCA score, which ranges from 1 to 30, provides a measure of cognitive function, with lower scores indicating greater cognitive decline. Our initial analysis revealed a weak-to-moderate positive linear correlation between MoCA scores and performance in noisy environments. This suggests that cognitive function may influence how well CI users perform in these challenging settings (Figure 1).
We also examined the relationship between cochlear health, as measured by electrocochleography-total response (ECochG-TR), and performance in noise. Similarly, we found a weak-to-moderate linear correlation between ECochG-TR and noise performance. An interesting pattern emerged when considering these correlations together: no patient with poor cochlear health (low ECochG- TR) performed well in background noise, and no patient with poor cognition (low MoCA score) excelled in noisy environments. This indicates that high cognitive function alone cannot compensate for poor cochlear health, and conversely, good cochlear health cannot make up for cognitive deficits when it comes to performing well in background noise.
Further analysis using a multivariate model that incorporated MoCA scores, ECochG-TR, and the interaction between these two factors (their product) explained 46-60% of the variance in noise performance. (1,2) Our findings suggest that both a good MoCA score (≥ 26) and a robust ECochG-TR value are required for excellent performance in noisy environments. In other words, good cochlear health is necessary but not sufficient for strong performance in noise – it must be paired with adequate cognitive function.
Interestingly, adding age to the model did not improve its predictive power, as there was significant collinearity between age and MoCA scores. We suspect that the impact of age on cognition and performance in noise is already captured within the MoCA score, rendering age an unnecessary additional variable in this context. This is of particular interest to our research team as we aim to understand better the various factors that contribute to cognition and their role in CI performance (R01DC020936-02; PI: Buchman).
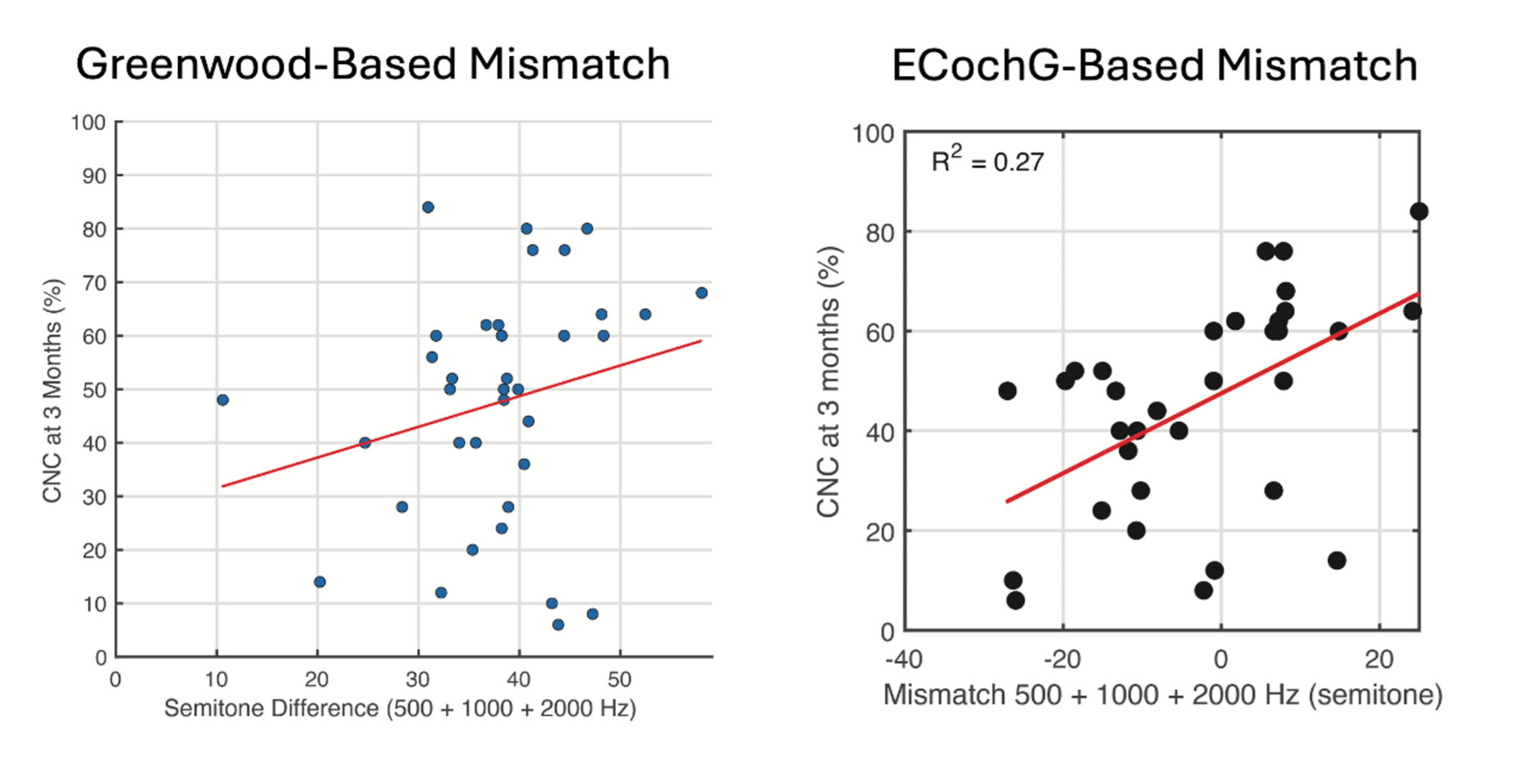
the ECochG-based mismatch and CI performance, suggesting that patients whose current manufacturer map deviates significantly from the ECochG-based map tend to perform worse than those whose maps align more closely with the ECochG-based recommendations.
Electrophysiologic personalized mapping of CI electrodes
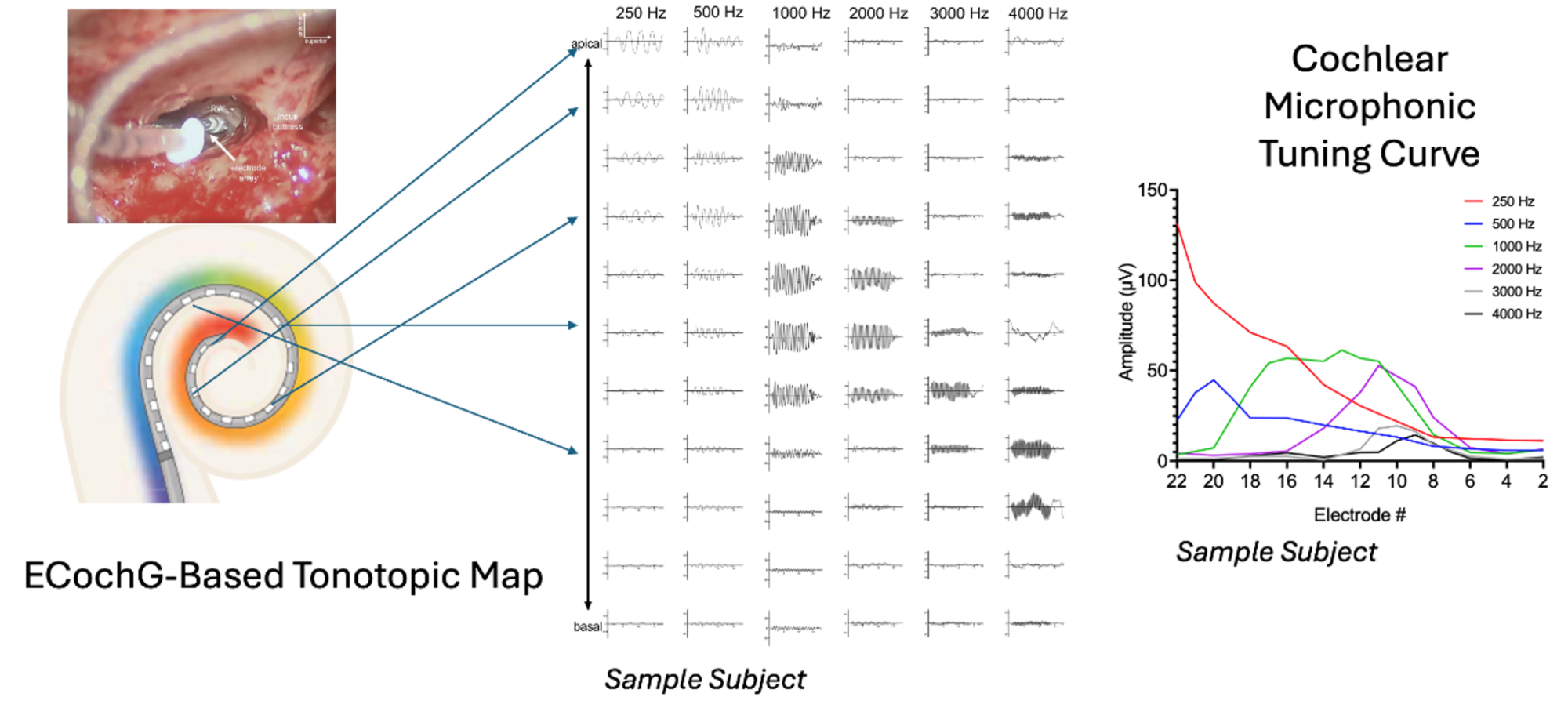
Modern CIs utilize electrode arrays of varying lengths inserted at differing depths within cochleae that vary in size. As the cochlea has a tonotopic arrangement, optimizing the frequency-to-place allocation for electrical stimulation provides an opportunity for improved performance. By doing so, the need for central adaptation and neural plasticity can be minimized, especially in post-linguistic adults.
There has been growing interest in applying established tonotopic maps, such as those described by Greenwood or Stakhovskaya et al., to postoperative imaging of electrodes to improve frequency allocation and place coding. Recently, acoustically-evoked electrocochleography (ECochG) has enabled us to pinpoint specific cochlear locations (i.e. place) where each acoustic frequency is most effectively processed. This advancement allows for the creation of personalized frequency allocation functions, thereby improving the precision of electrode mapping in CI programming (Figure 2). (3)
In our recent study involving 50 patients, we measured outer hair cell tuning curves using ECochG. We assessed the mismatch between the ECochG-based tonotopic map and the patient’s current map based on manufacturer settings, which typically follow a one-size-fits-all approach. (4) We found that patients with larger mismatches from the ECochG map tended to perform worse than those whose electrode mapping were more closely aligned with the ECochG map (Figure 3).
When we compared the mismatch between the patient’s current map (i.e. manufacturer default) and the Greenwood anatomical map, there was no correlation with performance. This suggests that the ECochG map is a better target for optimizing CI performance than that described by Greenwood or Stakhovskaya et al. This is likely best explained by the dynamic nature of the cochlear tonotopic map which varies by intensity of the stimulus. Furthermore, when we combined cochlear health (measured by ECochG-TR) with the mismatch between the patient’s current map and the ECochG-based tonotopic map, the model explained over 60% of the variability in performance.
The culmination of the described studies suggests that an ideal predictive model would incorporate cochlear health (ECochG-TR), cognitive function, and frequency to place mismatch. Moving forward, our research will continue to explore how personalized mapping strategies, informed by ECochG, can be further refined to enhance CI performance, particularly in challenging auditory environments.
References
- Walia A, Shew MA, Kallogjeri D, et al. Electrocochleography and cognition are important predictors of speech perception outcomes in noise for cochlear implant recipients. Sci Rep. Feb 23 2022;12(1):3083. doi:https://doi.org/10.1038/s41598-022-07175-7
- Walia A, Shew MA, Lefler SM, et al. Factors Affecting Performance in Adults With Cochlear Implants: A Role for Cognition and Residual Cochlear Function. Otol Neurotol. Dec 1 2023;44(10):988-996. doi:https://doi.org/10.1097/mao.0000000000004015
- Walia A, Ortmann AJ, Lefler SM, Holden TA, Puram SV, Herzog JA, Buchman CA. Electrocochleography-based Tonotopic Map: I. Place Coding of the Human Cochlea with Hearing Loss. Ear Hear. Accepted. 2024 July
- Walia A, Shew MA, Varghese J, et al. Electrocochleography-Based Tonotopic Map: II. Frequency-to-Place Mismatch Impacts Speech- Perception Outcomes in Cochlear Implant Recipients. Ear Hear. Jun 17 2024;doi:https://doi.org/10.1097/aud.0000000000001528

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.