Standardizing data collection in ME/CFS research through Common Data Elements is a crucial step toward improving diagnosis, advancing treatments, and fostering collaboration across studies to accelerate progress in understanding this complex disease
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a serious, debilitating, and underdiagnosed chronic disease characterized by profound fatigue, unrefreshing sleep, and post-exertional malaise. (1) Despite decades of research, the etiology and pathogenesis of ME/CFS remain unclear. The complexity and heterogeneity of the disorder pose significant challenges for researchers working to identify its underlying mechanisms and develop effective treatments. (1-2) In addition, variability in the diagnostic criteria, assessment methods, and outcome measures used across studies impedes researchers’ ability to compare findings. (2) Adopting Common Data Elements (CDEs) in ME/ CFS research is critical in addressing these challenges.
What are common data elements?
According to the National Library of Medicine, a CDE is a ‘standardized, precisely defined question, paired with a set of allowable responses, used systematically across different sites, studies, or clinical trials to ensure consistent data collection.’ (3) Approved CDEs are stored in the NIH Common Data Elements Repository, which provides detailed definitions, classifications, and guidelines for various data elements.
Development of initial ME/CFS CDEs
The initial ME/CFS CDEs released in February 2018 were developed collaboratively by the National Institute of Neurological Disorders and Stroke (NINDS) and the Centers for Disease Control and Prevention (CDC). These CDEs were categorized into four levels of recommendation: Core, Supplemental–Highly Recommended, Supplemental, and Exploratory, with Core CDEs representing essential information required for all ME/ CFS-specific studies. (4-5) More information on the classification of CDEs can be found on the NINDS website.
In 2022, the ME/CFS Core CDEs were revised to focus on four key objectives:
- Reducing the time needed to complete all the items;
- Eliminating optional or alternative elements;
- Removing items that require clinician assessment; and
- Incorporating items essential for evaluating major ME/CFS case definitions.
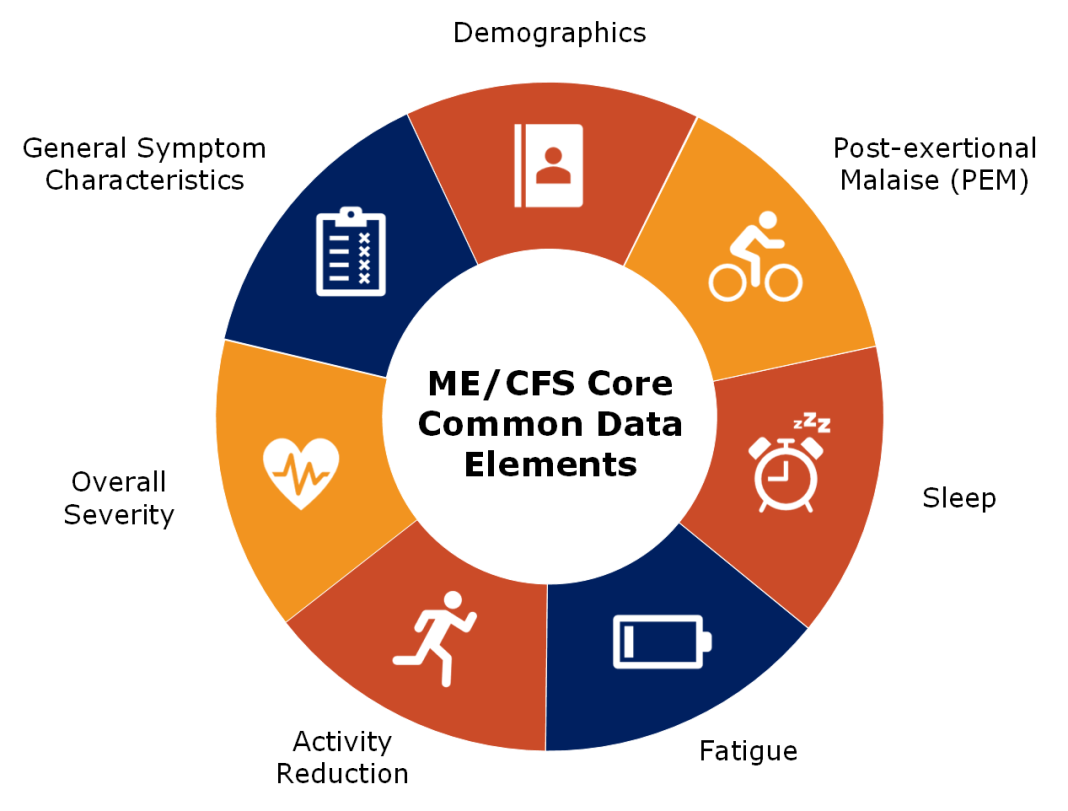
These efforts culminated in developing Version 2 of the ME/CFS Core CDEs. The updated set includes 68 items, and a breakdown of the domains can be seen in the accompanying diagram.
A complete list of ME/CFS CDEs can be found on the NINDS website: NINDS | ME/CFS Data Standards.
The next phase of ME/CFS CDE development
Since the release of Version 2 of the ME/CFS Core CDEs in 2023, the ME/CFS CDE Oversight Committee has recognized areas for continued improvement. With funding from the National Institutes of Health (NIH), the ME/CFS Research Network’s Data Management and Coordinating Center is supporting an initiative to revise and expand CDEs across additional key domains of ME/CFS. This effort brings together a multidisciplinary group of clinicians, researchers, and persons with lived experience, ensuring that the recommendations are both scientifically rigorous and patient-centered.
In Year 1, three working groups comprising around six members are working to develop CDEs related to:
- Post-exertional malaise:
- A hallmark symptom of ME/CFS. Understanding its triggers and duration could provide insights into disease mechanisms and help define subgroups of patients with different severity levels.
- Overall disease severity:
- Effectively and accurately measuring the impact of ME/CFS on daily functioning and quality of life will help track disease progression.
- Cognitive assessments:
- Accurately assessing cognitive impairments is crucial for understanding the neurological aspects of ME/CFS and identifying tailored interventions that address the cognitive challenges patients face.
The primary objective is to submit newly defined or updated ME/CFS CDEs for these three domains to NINDS. Outreach efforts will promote awareness and encourage integration into future ME/CFS studies. The extent to which these CDEs will be used in future NIH- or CDC-sponsored research will depend on their classification (e.g., Core vs. Exploratory), which determines their expected use in funded studies.
The second year of this effort will build on the progress made in the first year, expanding the ME/CFS CDEs to additional domains. The selection of these new domains will be guided by insights gained during the first year and the resulting CDEs.
Shaping the future of ME/CFS
The adoption of CDEs in ME/CFS research is a critical step toward improving the standardization and quality of data collection and analysis. ME/CFS investigators must utilize CDEs to ensure that their research contributes to a unified body of knowledge that can be compared and combined across studies to deepen our understanding of ME/CFS etiology and pathogenesis. As the field continues to evolve, ongoing refinement and expansion of the ME/CFS CDEs will be essential to address emerging challenges and incorporate new scientific insights. Through these efforts, we can move closer to unraveling the complexities of ME/CFS and developing effective interventions to prevent, treat, or improve disease.
Funders: U.S. National Institutes of Health: National Institute of Neurological Disorders and Stroke; National Institute of Allergy and Infectious Diseases; National Heart, Lung, and Blood Institute; National Center for Complementary and Integrative Health; and National Institute on Alcohol Abuse and Alcoholism.
Acknowledgment: This work was supported by the National Institutes of Health (NIH) under award number U24NS105535. The contents are solely the authors’ responsibility and do not necessarily represent the official views of NIH or RTI International.
References
- Centers for Disease Control and Prevention (CDC). (2024, May 10). IOM 2015 diagnostic criteria. CDC- Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
- Haney, E., Smith, M. E., McDonagh, M., et al. (2015). Diagnostic methods for Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome: A systematic review for a National Institutes of Health Pathways to Prevention workshop. Annals of Internal Medicine, 162, 834-840. [Epub 16 June 2015]. doi:10.7326/M15-0443
- National Institutes of Health. (n.d.). NIH Common Data Elements (CDE) repository. U.S. National Library of Medicine. https://cde.nlm.nih.gov/home
- National Institute of Neurological Disorders and Stroke (NINDS). (n.d.). Glossary. NINDS Common Data Elements. https://www.commondataelements.ninds.nih.gov/glossary
- National Institute of Neurological Disorders and Stroke (NINDS). (n.d.). Myalgic encephalomyelitis/ chronic fatigue syndrome. NINDS Common Data Elements. https://www.commondataelements.ninds.nih.gov/Myalgic%20Encephalomyelitis/Chronic%20Fatigue%20Syndrome