With current strategies proving inadequate, what needs to be done is to further the research into detecting, treating, and controlling ovarian cancer
Specifically, ovarian cancer develops when malignant ovarian cancer cells proliferate on the surface of the ovary and directly migrate/exfoliate to the peritoneal cavity where they attach to structures such as the omentum.
Women at early stages of tumor growth on the omentum do not display specific symptoms and hence the tumors remain unnoticed until tumor growth is extensive.
Because the ovarian tumor cells do not metastasize via the blood stream early detection of mutant cells in serum samples is not feasible with current methods. More sensitive assays and specific markers of ovarian cancer are needed.
Ovarian cancer is currently treated by the use of cytotoxic drugs cisplatin and paclitaxel that impair cell division by disrupting microtubule functions that control cell division and by inhibiting factors that control DNA repair.
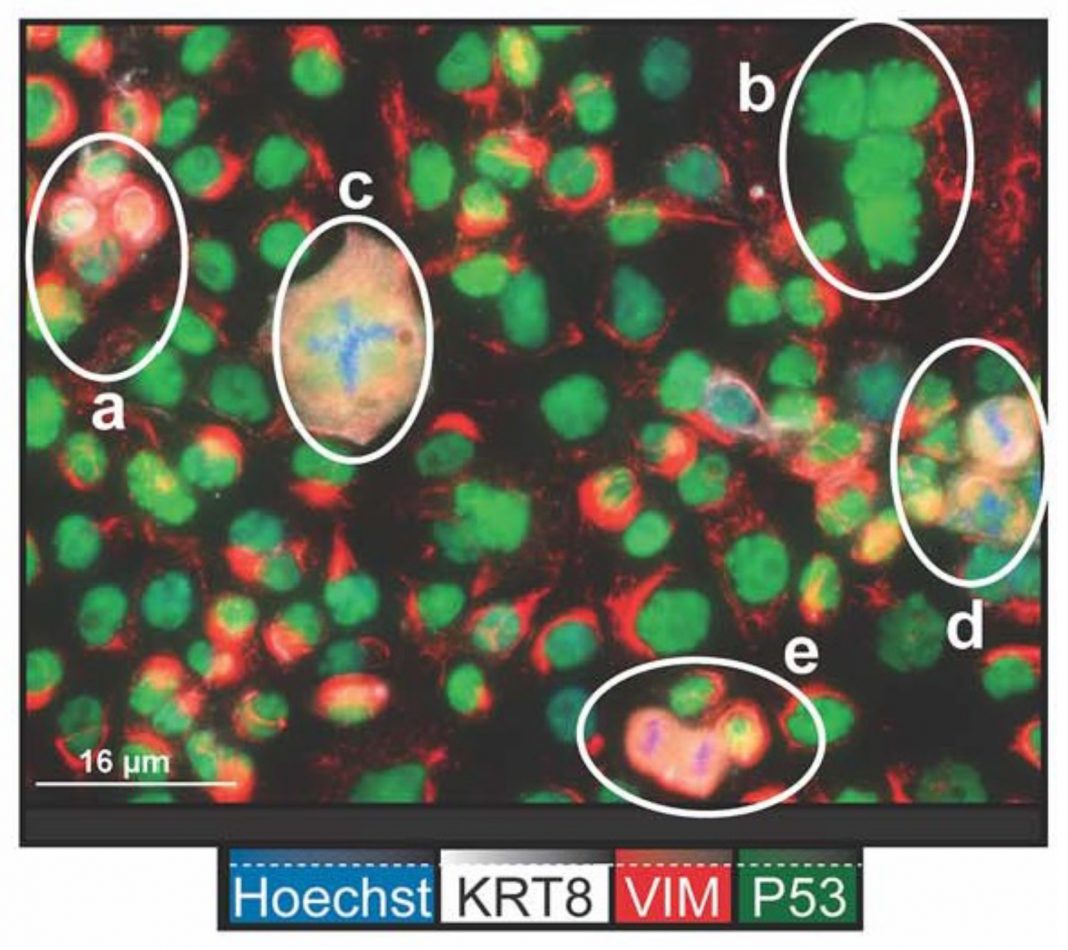
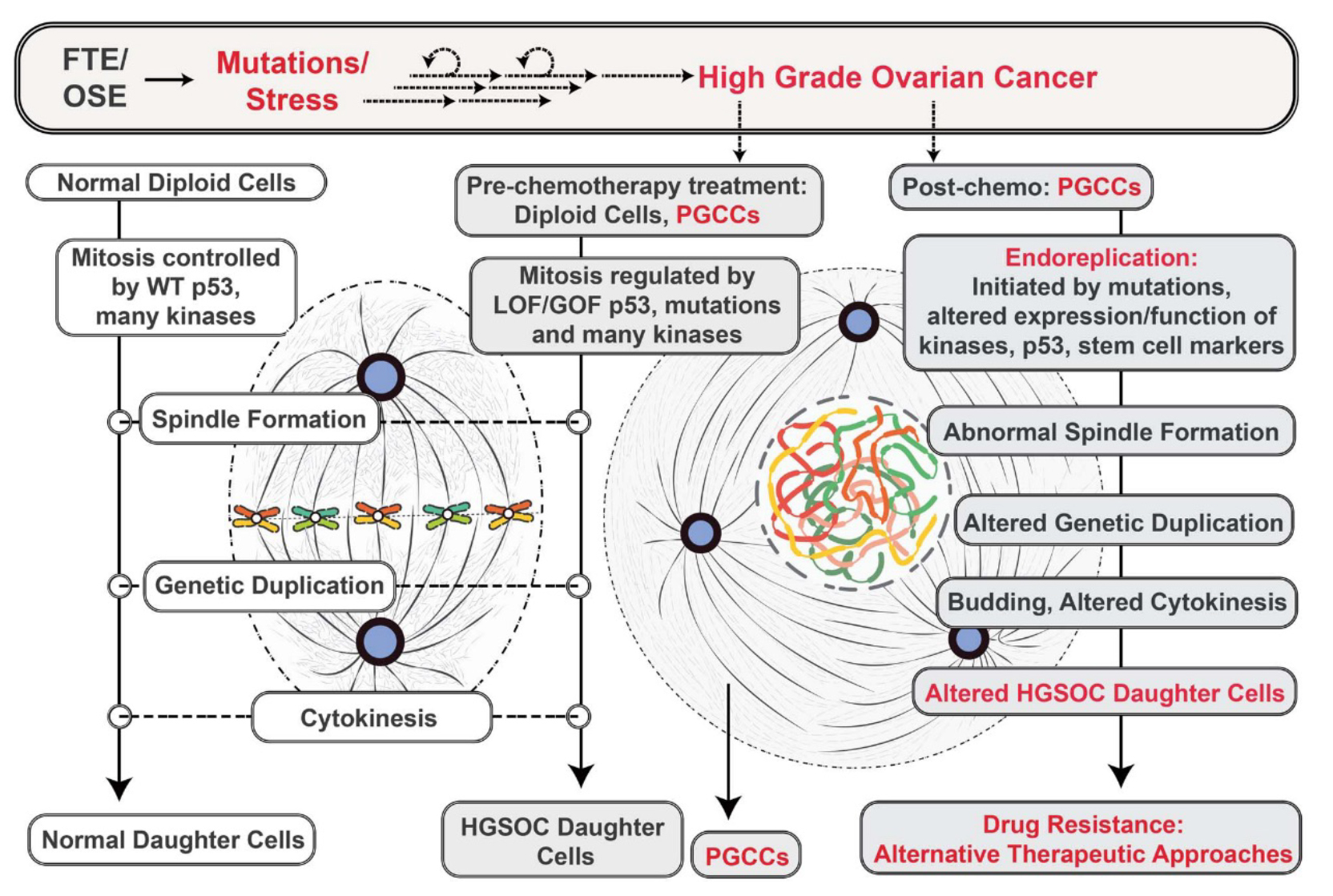
The tumor cells not only undergo cytotoxic stress leading to cell death but also evade cell death by the generation of specific daughter cells that do not respond to the toxic stress. These specific tumor cells known as polyploid giant cancer cells (PGCCs) give rise to daughter cells that by DNA alterations become resistant to the cytotoxic drugs and stress (1).
New targets of detection
Although most ovarian cancer tissue remains in the peritoneal cavity, ovarian cancer cells can, but rarely metastasize to breast and other tissues. Although PAX8 has been used to identify metastatic ovarian cancer cells, it is not specific. A recent study has documented that a protein known as SOX17 is expressed selectively and at elevated levels in ovarian cancer tissue and at metastatic sites and is more specific than PAX8 (2).
What are these factors?
PAX8 (Paired BOX 8) is a DNA Binding transcription factor that has been used to identify primary tumor cells of multiple origins, including not only ovarian but also breast and adrenal others. Hence it lacks tissue specificity (2).
SOX17 (SRY-box transcription factor) is a nuclear protein that is critical in regulating genes involved in the embryological development of many tissues. Recent studies have shown that it is also highly expressed in high-grade serous ovarian cancer cells (HGSOC) and in some esophageal squamous cell carcinoma (ESCC) cells that are sensitive to the cytotoxic drugs (2, 3).
What does SOX17 do?
A recent study shows that high expression of SOX17 inactivates the transcription of specific genes involved in DNA repair and tissue homeostasis. These genes include BRCA1, BRCA2, RAD51, SIRT1 and p21CIP1. By preventing DNA repair SOX17 sensitizes these tissues to the anti-cancer drugs such as cisplatin and paclitaxel that are used to disrupt cell division and lead to cancer cell death. Conversely, methylation of SOX17 permits high expression of DNA repair genes and resistance to DNA damage-inducing factors (3).
Ovarian cancer, PAX8 and SOX17
Routine treatment for controlling ovarian cancer includes physical removal of tumor tissue from the peritoneal cavity combined with administration of cisplatin and paclitaxel.
Recent studies have shown that ovarian cancer not only expresses high levels of PAX8 but also SOX17, providing 2 markers of high-grade serous ovarian cancer. This helps explain the sensitivity of high-grade ovarian cancer to the cytotoxic drugs and the use of these drugs in ovarian cancer treatment. The recent documentation of SOX17 expression in ovarian cancer is a key step in understanding one underlying mechanisms by which the sensitivity of ovarian cancer to specific drugs is mediated (3).
However, cytotoxic drug treatments do not by themselves eliminate cancer cells and tumor growth. Recent studies have identified PGCCs that have unique properties to evade cytotoxic stress and exist in 37-50% of high-grade tumors. Specifically, when these cancer cells undergo stress, they enter a unique form of cell division known as endoreplication. The end result of this process is the generation of many daughter cells that by genetic rearrangements have become resistant to the cytotoxic drugs. PGCCs (4).
What are the solutions to controlling ovarian cancer?
The recent evidence documents that SOX17 is a key and quite specific marker of ovarian cancer (2). Therefore, it could become a highly useful predictor of ovarian cancer and used along with other tests to identify and verify the presence of malignant ovarian tissue (and a subset of ESCCs) at an early stage and for revealing the presence of metastatic ovarian cancer tissue.
Many recent studies are beginning to highlight the derivation and key role of PGCCs to ovarian cancer progression (4). PGCCs can be induced by many different specific cytotoxic treatments used in cancer cell prevention. These include irradiation, chemotherapy, hypoxia or viral-oncogene-induced senescence.
Therefore, we need to understand the underlying molecular mechanisms that regulate endoreplication, the process by which PGCCs survive and lead to drug-resistant daughter cells. With this knowledge, new approaches for controlling ovarian cancer could be developed.
Of note the process of PGCC endoreplication is similar to that which occurs in early embryogenesis and thus is a very fundamental biological process (4).
References
- Richards JS, Candelaria NR, Lanz RB. Polyploid giant cancer cells and ovarian cancer: new insights into mitotic regulators and polyploidy. Biol Reprod. (2021):1-12.
- Zhang X, Yao J, Niu N, Li X, Liu Y, Huo L, et al. SOX17:
A Highly Sensitive and Specific Immunomarker for Ovarian and Endometrial Carcinomas. Modern pathology. (2023) 36(2):100001-. - Kuo IY, Huang Y-L, Lin C-Y, Lin C-H, Chang W-L, Lai W-W, et al. SOX17 overexpression sensitizes chemoradiation response in esophageal cancer by transcriptional down-regulation of DNA repair and damage response genes. J Biomed Sci. (2019) 26(1):20.
- Li X, Zhong Y, Zhang X, Sood AK, Liu J. Spatiotemporal view of malignant histogenesis and macroevolution via formation of polyploid giant cancer cells. Oncogene. (2023) 42(9):665-78.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.