It remains unclear whether boiling metal surfaces can still show surface tension, says Prof Dr-Ing. Jörg Volpp, Associate Professor at University West, Trollhättan in Sweden
Modern manufacturing methods involve thermal processes that heat metal materials to temperatures exceeding their boiling point. This is common in various techniques, such as welding, cutting, and additive manufacturing. Since the quality of the outcome is also defined by surface tension, gaining a better understanding of how these extreme conditions affect surface tension is highly desirable.
Surface tension
Attractive forces between the atoms or molecules within the surface of a liquid result in forces parallel to the surface, which we call surface tension. Surface tension is temperature-dependent and generally decreases at higher temperatures due to reduced attractive forces. The typically assumed linear decrease of surface tension with temperature seems unlikely to occur when approaching boiling temperature. Non-linear effects are known to appear, e.g., the rate of vapourisation. It also implies that surface tension disappears at some point.
However, at very high temperatures, there is only scarce data available due to challenges of measurements and modelling. Producing a metal surface at a homogeneous boiling temperature is difficult, and the extreme conditions can also influence the measurements themselves. Two experimental approaches were developed and compared to simulation results to better surface tension. boiling temperature
Surface tension measurements
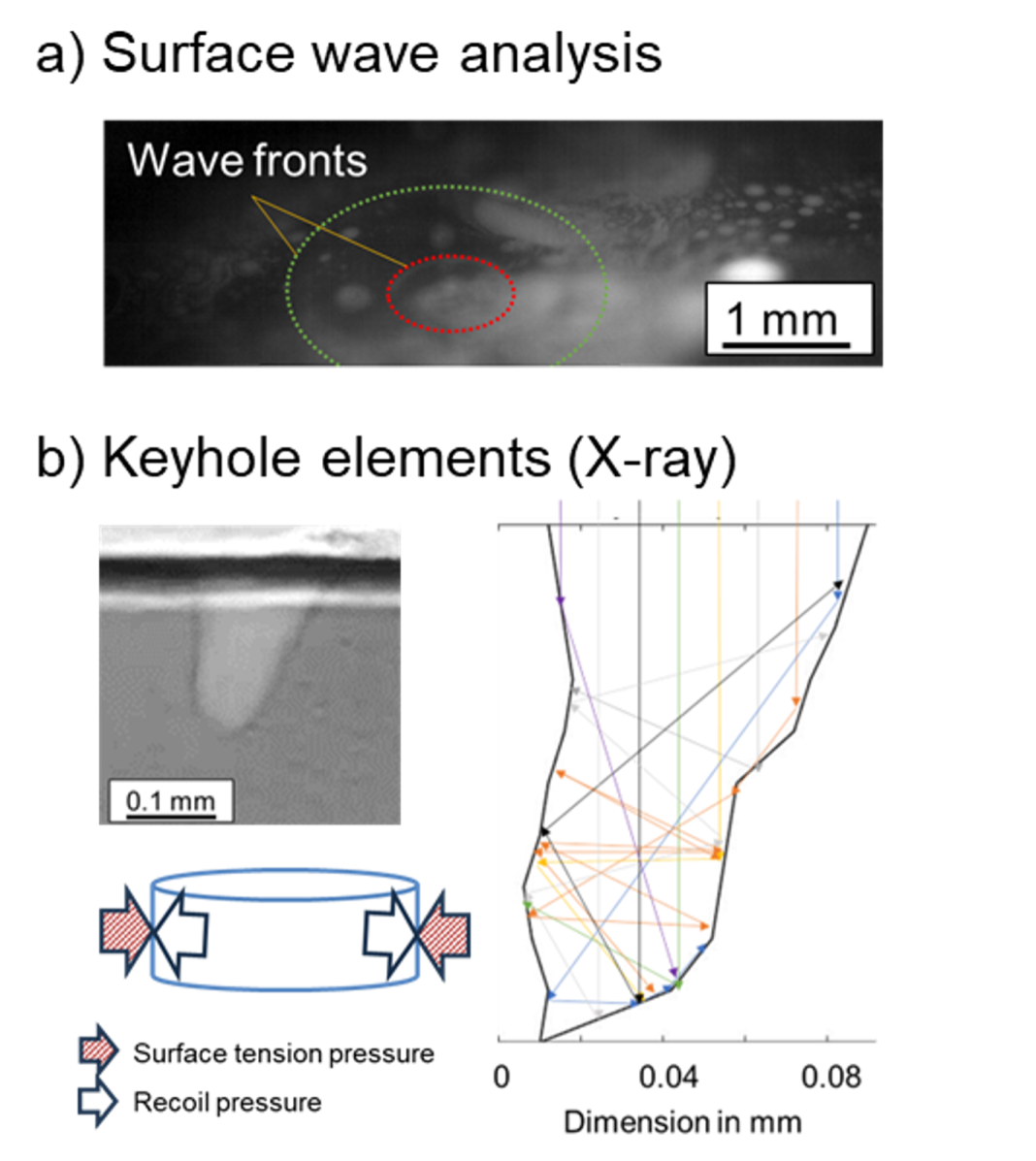
Two possibilities to create boiling surfaces were tested, namely surface wave analysis on laser-induced melt pools and laser-induced vapour channel measurements (Fig. 1).
Surface waves travelling on a liquid surface are related to the acting surface tension. Therefore, a laser beam was used to create high-temperature metal surfaces and induce incidents that create waves. (1) When deriving the frequencies and wavelengths of the induced waves from high-speed imaging videos, surface tension values were calculated (Fig. 1a). Parallel temperature measurements could link the surface tension to the actual temperature.
Vapour channels in metals appear when the energy input of a heat source is high enough to induce extensive vapourisation. This occurs, for example, in laser deep penetration welding. Inside the vapour channel, mainly the recoil pressure from metal ablation on the surface acts against the surface tension pressure of the surrounding melt (Fig. 1b). The existence of the vapour channel indicates that the surface tension does not disappear entirely even at temperatures above boiling temperature.
At Argonne National Laboratory’s synchrotron facility and the X-ray setups at University Stuttgart and Osaka University (2,3), vapour channels were recorded during laser irradiation using X-ray radiography. A ray tracing method was used to estimate the temperatures on the vapour channel walls and derive the local recoil pressure. Using the fact that the surface tension needs to counteract this pressure, the surface tension could be derived.
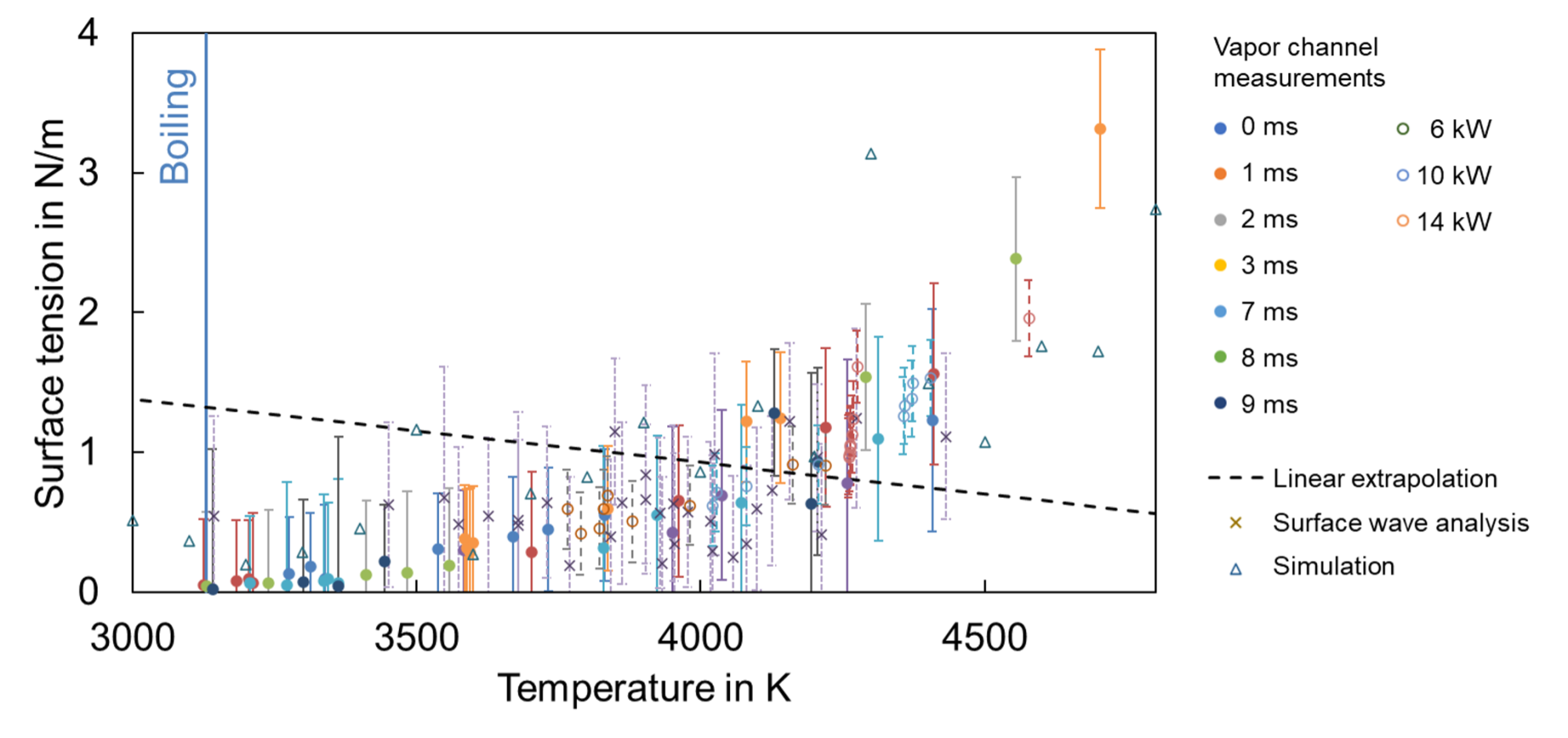
Surface tension above boiling temperature
Surface waves, vapour channel measurements for surface tension derivation, and simulation results show that surface tension still exists above boiling temperature (Fig. 2). However, the typically assumed decreasing trend is not visible. Instead, surface tension seems to increase again at increasing temperatures above the boiling point. This seems understandable when looking at the vapour channel. At surfaces with the highest energy input and temperatures, the vapour channel has the lowest diameters, meaning that the acting surface tension must be very high to counteract the intense recoil pressure.
Several explanations for the unexpected trend are possible. Due to the loss of atoms at extensive boiling, the surface will not appear smooth but show holes where atoms leave the surface as vapour. A hole in the surface denotes an increased surface. According to the common theories about surface tension, an increased surface increases the surface tension. Therefore, the sole surface geometry might contribute to the observed trend.
In addition, an atom that leaves a hole in the surface leads to a loss of bonding forces of the surrounding surface atoms. This can lead to increased forces on the remaining neighbours and to the atoms of lower layers. Those can result in increased forces parallel to the surface, which in turn increases the overall surface tension.
A simplified model was developed that calculates the arrangement of liquid iron atoms in a cube lattice based on a simple Lennard-Jones interaction model. The resulting atom positions and bonding forces in the surface layer indicate the acting surface tension. Although simplifications were implemented (1), the experimentally measured trend could be reproduced. This suggests that the model contains the effect leading to increased surface tension. A possible effect could be that attractive forces increase again at very high temperatures. (1)
The newly created knowledge can help to develop more energy-efficient material processing techniques to reduce the high energy need of the manufacturing industry.
Acknowledgements
Vetenskapsrådet (The Swedish Research Council), SMART – Surface tension of Metals Above vapoRization Temperature (2020–04250)
References
- Volpp, J. (2024). Surface Tension Estimation of Steel above Boiling Temperature. Applied Sciences, 14(9), 3778.
- Volpp, J., Sato, Y., Tsukamoto, M., Rathmann, L., Möller, M., Clark, S. J., … & Klingbeil, K. (2024). The surface tension of boiling steel surfaces. Results in Materials, 100583.
- Volpp, J., Zaiss, F., Hagenlocher, C., & Graf, T. (2024). Surface tension derivation from laser-generated keyholes. Journal of Laser Applications, 36(3).