Markus Mandler, Founder and CEO of Tridem Bioscience, explains how the company’s cutting-edge vaccine platform is revolutionising treatment approaches and vaccines for non-communicable diseases (NCDs)
Vaccines are one of the best health investments money can buy. One has only to look at how transformative vaccines were during the COVID-19 pandemic to appreciate how world- changing they can be. Currently, vaccines’ full benefits are only truly realised when targeting pathogens, which are innately immunogenic due to their foreign nature. Non-communicable diseases (NCDs), otherwise known as chronic diseases, usually lack immunogenic targets. Treating NCDs with vaccines relies instead on targeting self-antigens, which are generally well tolerated by the immune system.
The potential global health benefits would be staggering if efficacious vaccines could be developed for NCDs, which cause a whopping 76% of deaths globally and over 60% of all disability- adjusted life-years (DALYs).
Decades of research, however, have made precious little headway in this endeavour, primarily due to an inability to induce the strong immune responses needed to overcome tolerance to self-antigens. However, thanks to Tridem Bioscience and the NEXGEN-PD project, we may now be at the turning point when concerning vaccines for NCDs.
The secret solution: Skin
Tridem Bioscience is a small private biotech company based in Vienna, Austria, led by venerated scientists with extensive backgrounds in vaccine development. Tridem Bioscience has developed a proprietary and revolutionary novel vaccine platform termed WISIT (Win the Skin Immune System Trick), capable of achieving truly extraordinary immune responses against even poorly immunogenic self-antigens.
Conventional vaccines are usually applied intramuscularly or subcutaneously. But muscle and fat have not evolved to have a strong immune presence – how often are immune responses naturally needed in muscle and fat? The true immune goldmine is the interface to the outside world, where constant cuts and scrapes necessitate the rich presence of potent immune cells capable of readily initiating strong and rapid immune responses.
Whilst administrations of conventional vaccines to the skin have been explored before, conventional vaccines are not generally formulated to leverage skin immune cells. The WISIT platform, on the other hand, is formulated specifically for intradermal administration, finally unleashing the hitherto untapped potential of the skin immune system. To extraordinary effect!
How much difference does it make?
Parkinson’s disease is the first disease WISIT is being tested upon, though it will certainly not be the last. Parkinson’s disease is characterised by an abundance of misfolded alpha-synuclein (aSyn), a self-antigen with limited immunogenicity. Reducing aSyn is clearly associated with reduced Parkinson’s disease severity, though current means to reduce aSyn are greatly limited in the amount they can clear.
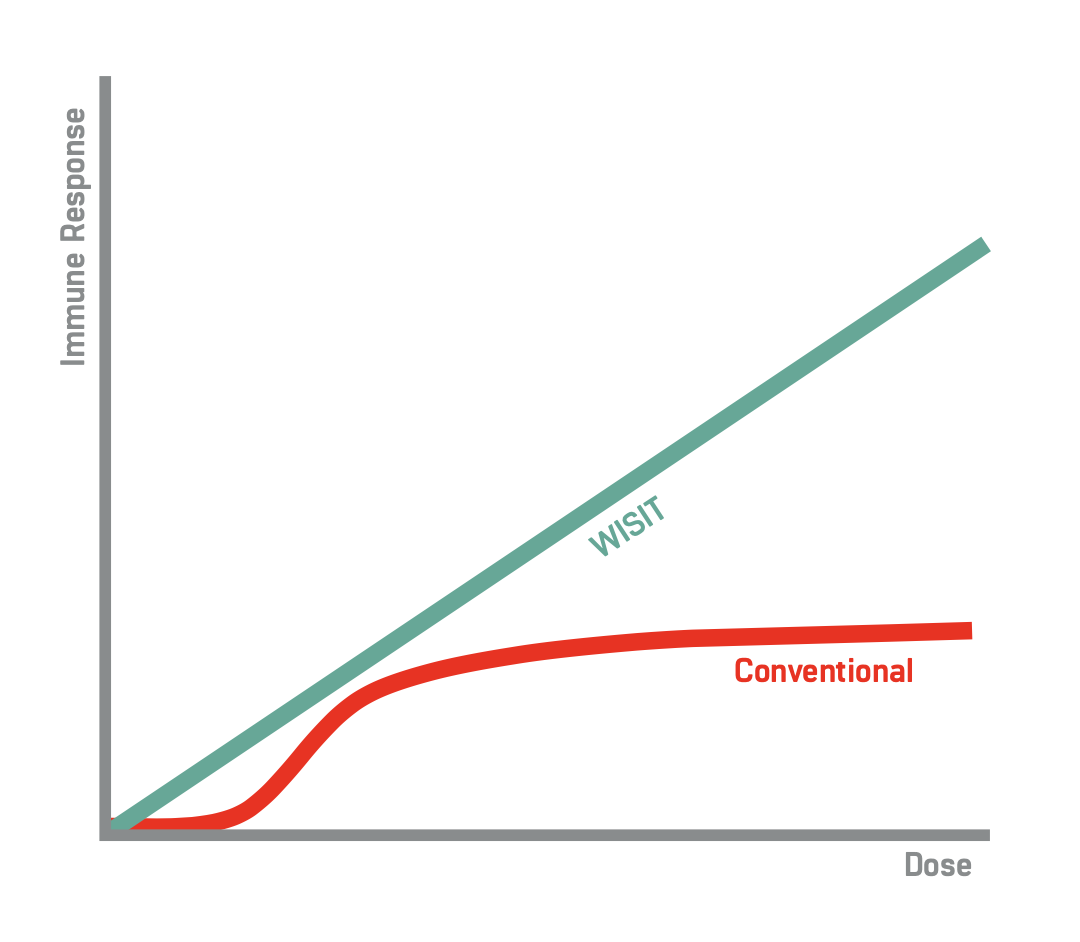
Preclinical experiments targeting aSyn in mice have compared WISIT side-by- side with a conventional Parkinson’s disease vaccine and shown astonishing improvements. At the conventional vaccine’s optimal dose, WISIT already demonstrates an x3-fold increase in antibody production and far higher quality antibodies to boot (see the evidence here).
Incredibly, early results from the NEXGEN-PD project show that this improvement can be increased many-fold further still, simply by increasing the dose! Conventional vaccines are dose-limited; increasing the dose yields no additional benefits. WISIT, on the other hand, uniquely provides continuously stronger immune responses as the dose increases. At the highest tested dose (see representative figure), a staggering x10-fold improvement is seen!
Vaccines for non-communicable diseases: What about the cost?
It might be presumed that such improvements must require increased cost or complexity of vaccines to achieve. In fact, the reverse is true, and these extraordinary improvements to immunogenicity result from a more economical and straightforward vaccine design!
Conventional vaccines require large amounts of adjuvants to be delivered alongside the vaccine to achieve an effect (attempting to overcome the sparse immune landscape of muscle and fat). WISIT, on the other hand, requires little to no additional adjuvant, reducing the cost of vaccine manufacturing by at least 30%. Considering even conventional vaccines are already highly cost-effective and widely applied in developing countries, WISIT would offer incredibly accessible NCD treatment for the entire world!
Where are we now, then?
The NEXGEN-PD project represents the critical next step to bringing WISIT from the lab to the clinic. Coordinated by Tridem Bioscience with support from Modus R&I and bringing together experts from across Europe, NEXGEN- PD is a Horizon Europe research project (Grant 101080267) focused on translating Tridem’s preclinical findings into first-in-man clinical trials to demonstrate the safety and immunogenicity of WISIT.
NEXGEN-PD additionally explores the possibility of combining WISIT with a novel non-invasive theragnostic assay developed at Paracelsus Medical University that can detect aSyn in blood or urine. Combining improved new early diagnostics with new effective treatment, NEXGEN-PD heralds a new era for treating Parkinson’s disease and kick-starts the transition to a new era of treatments for all NCDs. To learn more about the NEXGEN-PD project and receive updates on our progress and results, follow us on LinkedIn, check out our website, subscribe to our newsletter, or email us at Nexgen@modus.ltd.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.