Satyajit Hari Kulkarni and Christopher H. Contag from the Institute for Quantitative Health Science and Engineering focus on engineered endosymbionts, which they argue is a paradigm shift in anticancer bacteriotherapy toward killing tumors from the inside out
Cancer overview
Cancer is a genetic disease that can be initiated and/or exacerbated by environmental factors leading to metabolic changes in cells that result in uncontrolled growth. Key genetic changes in cancer cells include altered expression of cancer-promoting genes (oncogenes) or tumor suppressor genes. These genes encode proteins that promote cellular proliferation by interacting with DNA (transcription factors), nuclear proteins that control cell division at the level of the cell cycle, or proteins that prolong cell survival by avoiding programmed cell death (apoptosis).
As cancers progress, mutations accumulate, and at later disease states, most cancers have mutations in multiple genes controlling multiple pathways, leading to the well-described hallmarks of cancer. (1-3) The dysregulated pathways that lie at the root of cancers are intracellular and intrinsic to the cancer cells. Still, subsequent changes to cells surrounding the cancer cells, the stroma or tumor microenvironment, can occur, which are also controlled intracellularly.
A significant challenge for developing effective cancer therapeutics is to infiltrate the immunosuppressive tumor microenvironment for drug delivery of therapeutic agents to the inside of cancer cells to control these dysregulated pathways. We have been developing a platform technology called engineered endosymbionts, which are inherently intracellular and can be designed to deliver therapeutic molecules.
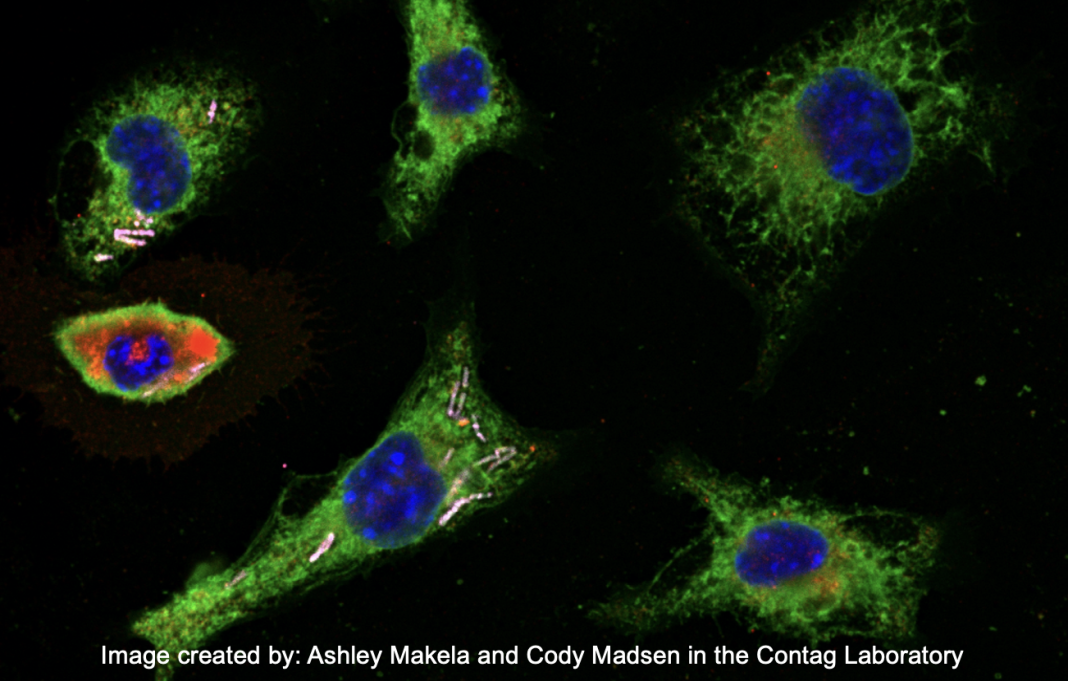
Engineered endosymbionts are based on innocuous, free-living bacteria that are engineered to enter into cells, persist in the cytoplasm of those cells, and then deliver therapeutic proteins inside of cancer and stromal cells in a tumor to alter their function. The use of bacteria as cancer therapies was proposed decades ago and is now a field referred to as bacteriotherapy. (4-7)
Bacteria as cancer therapeutics
The best example of a widely used bacteriotherapy is the treatment of bladder cancer with Bacillus Calmette- Guerin (BCG), a live attenuated form of Mycobacterium tuberculosis. (8) This bacterium is used internationally as a vaccine to prevent tuberculosis but has become the gold standard for treating high-grade and high-risk non-muscle-invasive bladder cancer. The use of BCG has been shown to reduce or prevent both recurrence and progression after initial transurethral resection of a bladder tumor. (8)
BCG is also used in veterinary medicine as an anticancer immunotherapy in equine sarcoids (9,10) using both live bacteria and cell wall preparations. Many consider bacteriotherapy as a version of immunotherapy that acts through local activation of the immune response against the tumor through simultaneous activation of antibacterial and antitumor defenses. (6) This mechanism can act on the immunosuppressive immune cells within the tumor microenvironment or through recruitment of circulating immune cells into the tumor.
Bacterial therapies have been injected locally into the tumor or delivered systemically, and are being developed as probiotics following oral delivery. (11) The mode of delivery of bacteriotherapies has recently been shown to affect the immune response, where intratumoral (IT) delivery alone promoted tumor growth through recruitment of immunosuppressive polymorphonuclear cells (PMN), while intravenous (IV) or a combination of IV and IT had antitumor effects. (12)
Bacteria have been used as live, attenuated, or genetically engineered bacteria for delivery of, for example, prodrug-converting enzymes that convert generally nontoxic chemicals into anticancer drugs. (13) More recently, bacteria have been used as a method to “tag” tumors for chimeric antigen receptor (CAR) T cells for tumor targeting and potentiating this cellular therapy. (14)
Additionally, bacteria have been designed to produce mammalian chemokines and camelid nanobodies to recruit immune cells and induce tumor regression in a localized manner. (15-18) In all of these approaches, the bacterial therapeutics are generally extracellular, outside of the tumor cells, and eliminate cancer cells from the outside in. The prefix endo-, in endosymbiont, refers to the bacteria dwelling inside the cell, and in the development of cancer therapies, the endosymbiont is engineered to interfere with, or control, the inner workings of the cell-targeting the root pathways of cancer or the key pathways in immune cell clearance of the tumor. (19) The paradigm shift in anticancer bacteriotherapy using engineered endosymbionts is based on using intracellular bacteria to functionally change cellular functions and kill cancer cells from the inside out.
Creating endosymbionts
Engineered endosymbionts are bacteria that generally do not naturally enter into and persist inside of mammalian cells but are engineered to have these functions. Engineered endosymbionts have been generated from several bacterial chassis organisms where the genetic systems have been well developed, such as the bacteria Escherichia coli (20,21) and Bacillus subtilis. (19)
As a chassis organism, B. subtilis has a number of features that make it particularly well-suited for engineering endosymbionts. Its genome has been sequenced and annotated, and since it has been utilized for industrial production of proteins, the genetic tools for engineering heterologous proteins to be secreted from this bacterium are well-developed. The observation that expression of a protein from Listeria monocytogenes, listeriolysin O (LLO) in B. subtilis, would enable the bacillus bacterium to enter mammalian cells, macrophages, and escape destruction by the cellular endosomes laid the foundation for this organism to be developed as an engineered endosymbiont. (19)
In the first demonstration of B. subtilis as an engineered endosymbiont, the mammalian genes encoding the transcription factors stat1 and klf6 were expressed as bacterial proteins and designed to be secreted into the cytoplasm of macrophages, enter the nucleus of the cell, bind to specific sites on the cellular DNA to drive genetic programs that would reprogram the tumour-associated macrophages into a proinflammatory state, and eliminate cancer cells. (19) This was the first demonstration of engineered endosymbionts reprogramming mammalian cells through the controlled expression of transcription factors.
Targeting cancer metabolism and immune function
The demonstration of guided cellular reprogramming by engineered endosymbionts set the stage for developing biological therapeutics that direct cellular functions from the inside out. This opened the possibility of controlling stem cells to become differentiated tissues, directing immune function and cancer therapeutics. Control of cellular function as a cancer therapy could act through engineered endosymbionts secreting proteins in cancer cells that control cell division, interfere with transforming or metabolic pathways, or in the cells that comprise the surrounding stroma, including immune cells, to eliminate the cancer. (19)
Ras is one of the most prevalent oncogenes that is mutated and aberrantly expressed in cancer. This oncogene controls many cellular pathways and is a target for many cancer therapies.
Additionally, p53 is one of the most commonly dysregulated tumor suppressor genes and controls the cell cycle. The pathways regulated by both of these tumor-associated genes could be targeted with proteins expressed from endosymbionts for the intracellular control of cancer growth.
The challenge for developing any therapeutic is effective delivery to the target sites where the therapy is needed. Bacteria, when injected intravenously, naturally accumulate in the tumor, presumably due to the leaky vasculature and hypoxic microenvironment of the tumor, often localizing to necrotic regions. Thus, bacteria have naturally solved the delivery problem due to their ability to persist in anaerobic and microaerobic environments. When engineered endosymbionts designed to control cellular function enter into mammalian cells and persist, the engineered endosymbionts can eliminate cancers from the inside out.
The future of engineered endosymbiont research
Control of cellular functions requires systematic control of many pathways, and at the genomic level, these pathways are regulated by transcription factors, i.e., proteins that bind DNA and direct gene expression. Bacterial genes are naturally polycistronic – they can express multiple genes encoding a variety of proteins from a single regulatory element. Also, these regulatory elements can be controlled by thermal energy, (22) small molecules, (19) quorum sensing, (23) and other signals that can be delivered to tissues in animals and humans.
Using these synthetic gene circuits, engineered endosymbionts can be designed to orchestrate a coordinated cellular response at the levels of tissues and organs, and be directed to express genes from outside the body with exquisite control. (22) One mechanism of control is to use the heat generated by alternating magnetic fields to activate genes in magnetotactic bacteria, which naturally have nanomagnets in organelles called magnetosomes. (24,25) These bacteria can be converted into magnetoendosymbionts, which will enable functional control of gene expression in the engineered bacterium with magnetothermal energy (22) and also provide a means of physically moving the bacteria with magnets.
These processes can then be imaged in living subjects using the magnetic particles as living contrast agents in magnetic resonance imaging (MRI) (26,27) and magnetic particle imaging (MPI). (28) Controlling cellular function in living animals and humans with signals transmitted from outside the body into specific target tissues in the body will enable regeneration of tissues to replace those that are lost or damaged, regulating the immune function, regenerating cells that are lost in degenerative diseases like Parkinson’s disease, and direct cellular systems of the body to treat many other injuries and diseases.
CLICK HERE for references

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.