European research reactors play a crucial role in producing medical radioisotopes using nuclear fuel and are transitioning to Low Enriched Uranium (LEU) to meet non-proliferation commitments and global demand. Jared Wight tells us how he and his colleagues are working to ensure a smooth conversion to LEU fuels
Nuclear medicine is critical in the fight against cancer. It relies on research reactors to produce the medical isotopes needed for treatment and diagnosis. As part of a global effort to reduce security risks, many reactors are transitioning from highly enriched uranium (HEU) to low-enriched uranium (LEU). This shift not only strengthens nuclear security but also ensures a stable supply of life-saving radioisotopes. In this way, fueling the fight against cancer takes on a new meaning, as the move to LEU supports both healthcare and global security.
Fueling the fight against cancer
Nuclear medicine plays a crucial role in every stage of the fight against cancer. Nuclear imaging is used to detect, diagnose, and assess various cancers. Therapeutic nuclear medicine uses specialized radioactive drugs to target specific tumors and destroy cancer cells. The application of nuclear medicine extends beyond fighting cancer. Medical radioisotopes are also used in countless other health procedures, from diagnosing neurological disorders to evaluating cardiac health.
Where do all these medical radioisotopes and radiopharmaceuticals come from?
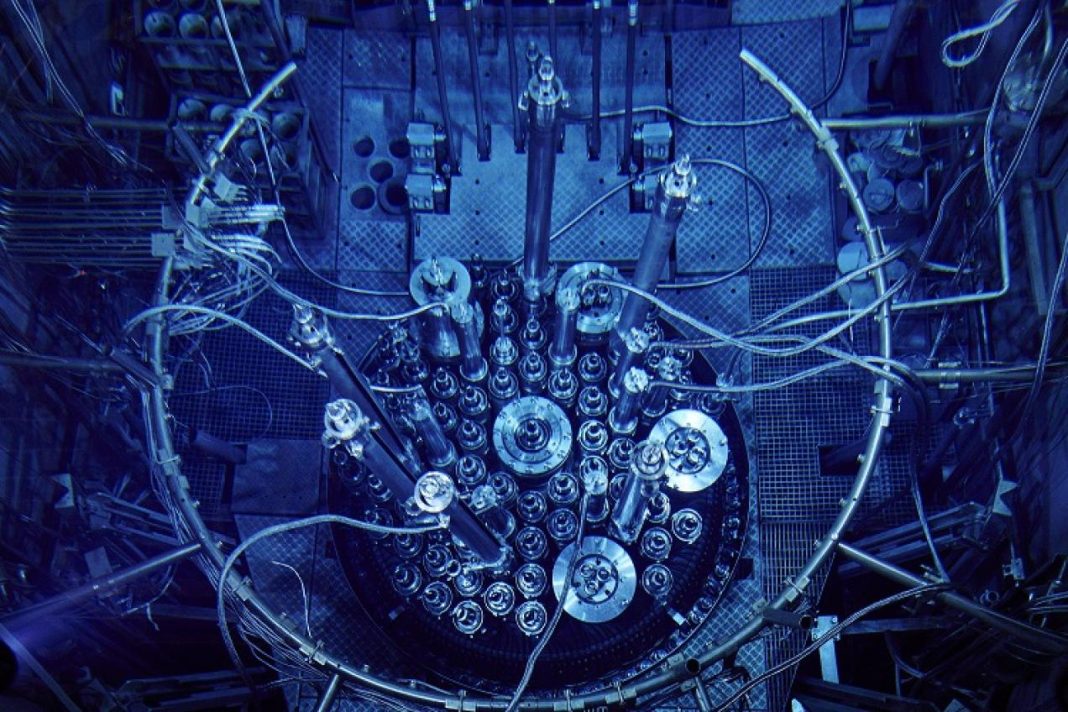
A significant proportion of the medical radioisotopes used around the world are produced in European research reactors, including 80% of the most essential radioisotope in nuclear medicine: technetium-99m (Tc-99m). This radioisotope is used in over 30 million medical diagnostic scans annually worldwide. It provides valuable insight on a wide range of medical conditions—from detecting tumors to assessing a brain after a stroke. Tc-99m is just one of many routinely used medical radioisotopes that can only be produced produced in significant quantities in high-performance research reactors (HPRRs). These research reactors were originally built to test new materials and conduct basic research. Over time, their usage has increasingly evolved to focus on the production of medical radioisotopes and advancing the ‘fight against cancer.’
Nuclear non-proliferation and fighting cancer
It is a seemingly unusual combination, but furthering nuclear security and cancer research go hand-in-hand. The reactors require a regular supply of nuclear fuel, but they are changing their fuel types to LEU to meet global non-proliferation commitments to ensure a safer world while continuing to deliver critical medical radioisotopes and other nuclear science. Three of the main HPRRs currently operating in Europe are using HEU fuels. However, the BR2 reactor at SCK CEN, the FRM-II reactor at the Technical University of Munich, and the High-Flux Reactor at Institut Laue-Langevin are planning to convert to LEU fuel that contains less than 20% uranium-235.
Converting these reactors to LEU fuels is necessary to strengthen the global nuclear non-proliferation regime and secure the future supply of critical medical radioisotopes. EU countries have made significant commitments to the international community to eliminate the use of HEU, especially when a LEU solution is feasible. This has led EU research reactors and the EU fuel fabricator, Framatome CERCA, to form a consortium to actively pursue LEU conversion together.
EU-QUALIFY
In the EU-QUALIFY project (European Qualification Approach for Low Enriched Fuel Systems for secure production supply of medical isotopes), Jared Wight and his colleagues are working to ensure a smooth conversion to LEU fuels to sustain the current global supply of radioisotopes – and to also ensure the development and production of newer drugs, such as lutetium-177 PSMA. This EU-funded research builds on two previous projects, HERACLES-CP and LEU-FOREvER, that developed and demonstrated novel, LEU-based fuels with higher density levels of uranium. The consortium pursuing LEU conversion has identified three types of LEU-based fuels: dispersed uranium-molybdenum (U-Mo), monolithic U-Mo, and high-density dispersed uranium-silicide (U3Si2) fuels.
The project team now aims to take these three fuels through a generic fuel qualification process, which is crucial to demonstrate how they will behave in a variety of conditions. The candidate LEU fuel must demonstrate through commercial-scale fabrication that conversion is feasible, and both irradiation experiments and neutronics calculations must confirm that the reactor-specific fuel performance targets can be met. Detailed post- irradiation examinations (PIE) are conducted in specialized facilities after the fuel is irradiated at prototypic reactor conditions. Safety is paramount throughout every stage. Detailed safety analyses of experiments and future LEU conversions are conducted to demonstrate that there will be no significant releases of radioactivity to workers, the public, or the environment in even the most unlikely of scenarios.
“The demonstrations and experiments conducted to date confirm that LEU conversion of our EU research reactors is feasible”, says Wight. “There are four irradiation experiments within EU- QUALIFY, two of which have been successfully performed. We’ve made some great progress within the project, but we still have much to do to complete it. This will ensure that we can continue to support our EU research reactor community with qualified options for a reliable LEU fuel supply.”
This work helps ensure that EU research reactors can continue operating and can continue to maintain the global medical radioisotope supply chain now and into the future.