Stoyanka Nikolova, Professor from Paisii Hilendarski Plovdiv University, discusses the potential of harnessing hybrid molecules for drug development and their possible application in addressing the clinical challenge of irritable bowel syndrome
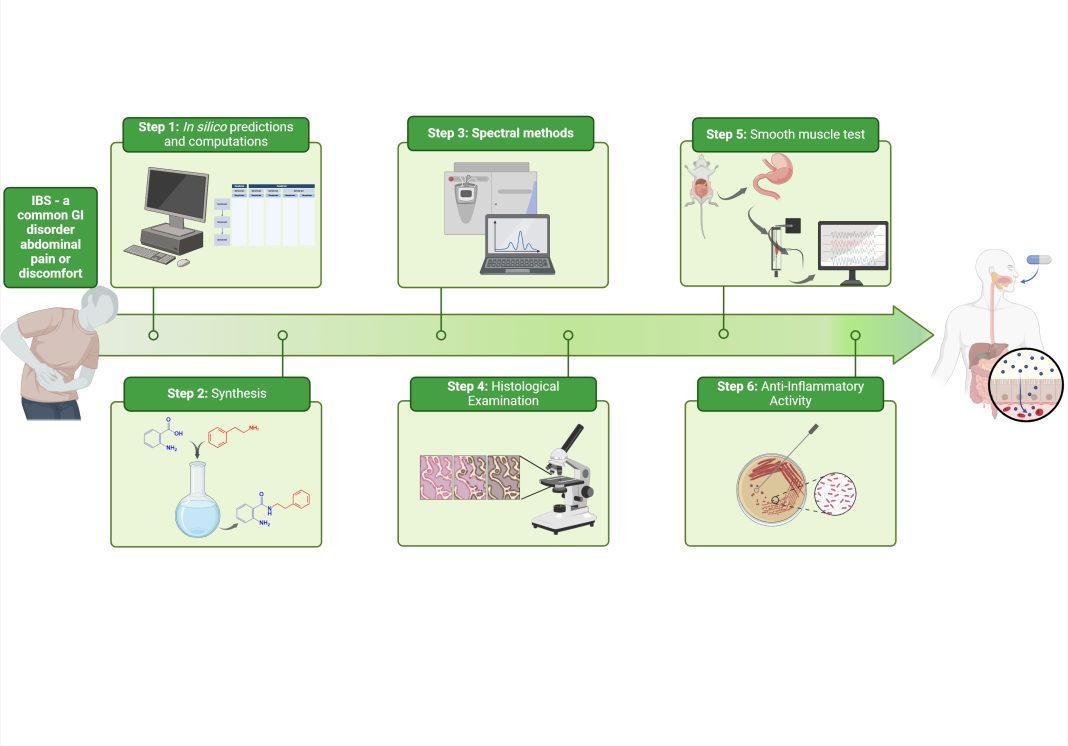
Irritable bowel syndrome (IBS) remains a clinical challenge. It’s the most common gastrointestinal disorder. IBS has no definitive treatment but could be controlled by non-pharmacologic management, eliminating some exacerbating factors such as certain drugs, stress conditions, and changes in dietary habits. Intestine peristaltic reflexes and sensory relays are significantly regulated by serotonin, predominantly found in intestine enterochromaffin cells.
Two lines of evidence support the abnormality of serotonin regulation in IBS. Serotonin release in plasma appears to be increased in diarrhoea-predominant IBS (IBS-D) or decreased in those with constipation-predominant IBS (IBS-C). Reduced normal mucosal serotonin and serotonin transporter immunoreactivity have been observed in both ulcerative colitis and IBS.
Synthesis of hybrid molecules: A strategic approach
The urgent need to address new health issues and enhance the effectiveness of treatment for a wide range of illnesses motivates the search for novel therapeutic agents, which is a current task in medicinal chemistry.
Significant attention has been attracted to the design and synthesis of hybrid molecules, combining two or more structural components from many bioactive molecules. They can improve biological activity and overcome medication resistance.
Synthesising hybrid compounds involves the deliberate combination of pharmacophores from several chemical entities to target numerous biological pathways or increase the potency of currently available medications. Recent research has shown that several synthetic pathways accomplish this frequently by utilising green chemistry ideas to reduce environmental effects and increase yield.
Biological evaluation: Promising therapeutic potential
The biological evaluation of synthesised hybrid molecules revealed significant therapeutic potential across several areas of medical need. We tested the compounds through a series of in vitro and ex vivo assays and found they demonstrated enhanced potency and selectivity compared to existing therapies.
We identified hybrid molecules with antispasmodic properties, which have shown potential in treating disorders such as irritable bowel syndrome (IBS). These compounds were designed to relax smooth muscle tissue, providing a novel treatment option for patients suffering from this chronic condition. The successful application of hybrid molecules in this context highlights their versatility and broad therapeutic applicability.
Moreover, we explored the antimicrobial activity of these hybrid molecules. Our studies demonstrated that several of our synthesised compounds exhibited potent activity against a range of pathogenic bacteria and fungi. These molecules were particularly effective against strains that have developed resistance to conventional antibiotics, highlighting their potential as novel antimicrobial agents. The dual-action mechanism of these hybrids allows for targeting multiple bacterial pathways simultaneously, reducing the likelihood of resistance development and offering a promising solution to the growing problem of antimicrobial resistance.
We also investigated the anti-inflammatory activity of our hybrid molecules. In various in vitro and ex vivo models of inflammation, these compounds significantly inhibited the production of pro-inflammatory cytokines and other mediators of inflammation. This activity indicates that our hybrid molecules may effectively treat chronic inflammatory conditions such as inflammatory bowel disease, rheumatoid arthritis and other autoimmune disorders. The ability to modulate multiple inflammatory pathways concurrently positions these hybrids as promising candidates for developing new anti-inflammatory drugs.
One of the most exciting outcomes was the discovery of hybrid molecules with strong antitumor activity. These compounds not only inhibited tumour growth effectively but also displayed a favourable safety profile, with reduced cytotoxicity toward healthy cells. This dual benefit underscores the potential of hybrid molecules to serve as more effective and safer alternatives to conventional chemotherapeutic agents.
Collaboration and funding: Catalysing progress
While our findings have laid a strong foundation for the therapeutic application of hybrid molecules, we recognise that further research and development are essential to bring these compounds to clinical use. This journey requires extensive collaboration with academic institutions, industry partners, and government agencies. By pooling our expertise and resources, we can expedite the translation of these promising molecules from the laboratory to the clinic.
External funding is also crucial to support the comprehensive research needed to optimise these hybrid molecules, conduct large-scale clinical trials, and ultimately deliver effective treatments to patients. We are actively seeking partnerships and funding opportunities to continue our work in this exciting field. We aim to establish a robust pipeline for developing hybrid molecules, contributing to the advancement of innovative therapeutics.
Conclusion: Harnessing hybrid molecules for drug development
Our research into the synthesis and biological evaluation of hybrid molecules has revealed their significant potential as novel therapeutic agents. The promising results achieved so far underscore the importance of continued exploration and development in this area. Through strategic collaboration and external funding, we are committed to advancing these compounds toward clinical application, hoping to improve treatment outcomes and address medical needs on a global scale.
We welcome all interested parties to join us in this collaborative effort. Together, we can achieve breakthroughs that will transform the future of medicine.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.