Do we need new antiretroviral drugs to treat HIV infection, and if so, what are the promising targets? Dr Eric O. Freed, Director of the HIV Dynamics and Replication Program at the National Cancer Institute in Frederick, Maryland, discusses these questions
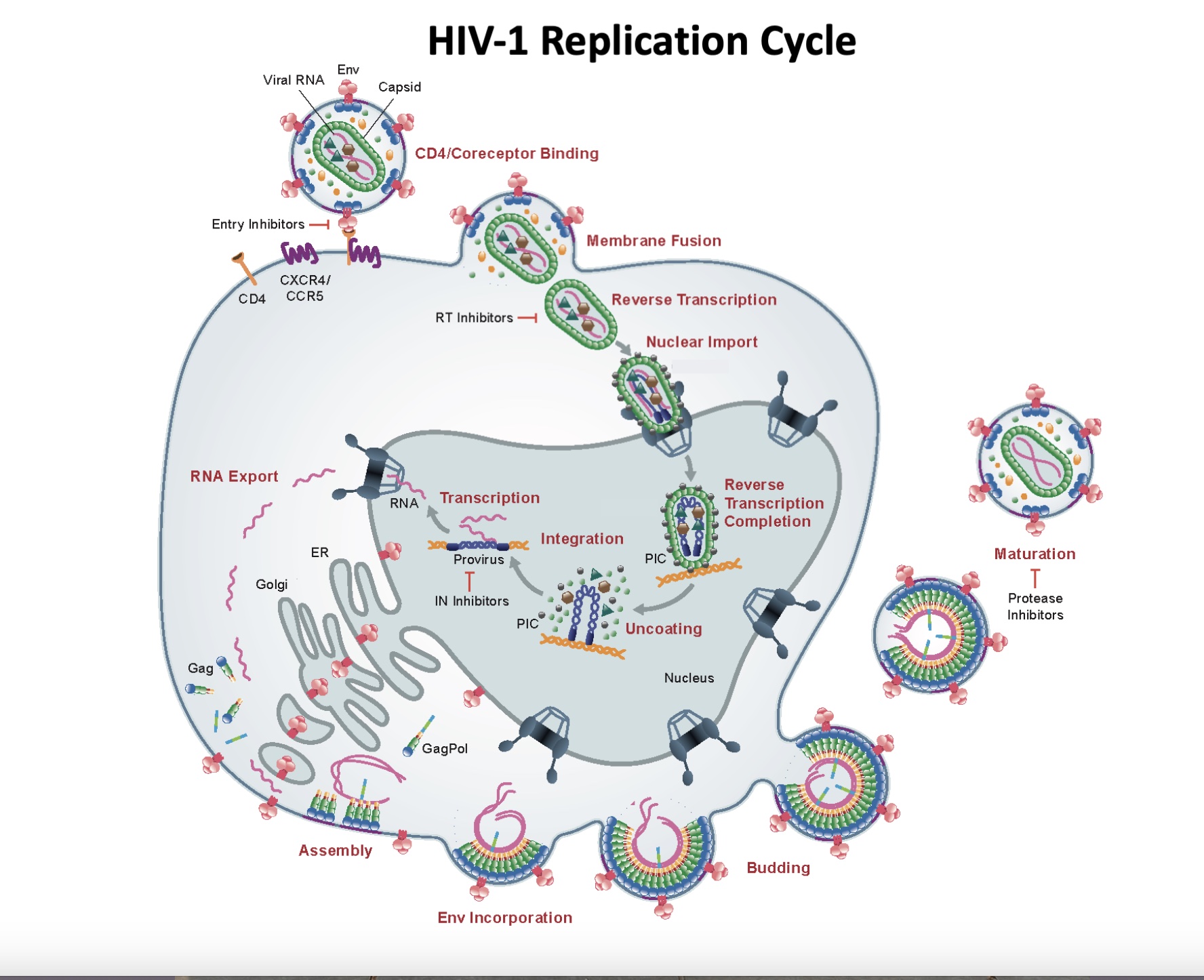
The HIV replication cycle (1) (see figure) presents numerous potential therapeutic targets, but only a few have been successfully exploited. HIV-1 infection begins when the viral envelope glycoprotein (known as Env) binds to its primary receptor (CD4) and coreceptors on the surface of a target cell, leading to a fusion between the lipid bilayer of the virus particle and the plasma membrane of the cell, allowing the viral core – which contains the viral RNA genome and key viral enzymes – to gain access to the target cell cytoplasm.
The viral RNA genome is then converted (or “reverse transcribed”) into double-stranded DNA by the viral enzyme reverse transcriptase (RT). During reverse transcription, the viral core is transported to the cell’s nucleus where ultimately the newly synthesized viral DNA is inserted (or “integrates”) into the host cell genome, a process catalysed by the viral integrase (IN) enzyme.
The integrated viral DNA is used to synthesize viral RNAs, which are translated into viral proteins and packaged into new viral particles. The assembly of new particles is a primary function of the Gag polyprotein precursor. After it is assembled, the virus particle buds off from the cell and “maturation” takes place. During maturation, the viral protease (PR) cleaves the Gag precursor, and the viral RNA genome is packaged into the viral core, together with RT and IN. The infection can now spread to new cells.
What antiretroviral drugs do we currently have?
Several major classes of drugs are currently available to treat HIV infection. The most successful antiretroviral drugs target the three viral enzymes: RT, IN, and PR. The first class of antiretroviral drugs were nucleoside RT inhibitors (NRTIs) that block the ability of RT to reverse transcribe the viral genome after infecting a cell.
When NRTIs were first used to treat HIV-infected individuals, some transient benefits were realized; however, the virus quickly became drug resistant. It was not until additional classes of inhibitors were developed – the non-nucleoside RT inhibitors (NNRTIs) and PR inhibitors (PIs) – that these drugs could be administered as combination antiretroviral therapy (cART) and sustained clinical benefits were achieved. In the years following the implementation of cART the number of deaths from HIV infection declined markedly.
In the ensuing years, other classes of antiretroviral drugs were developed, most notably the IN strand-transfer inhibitors (INSTIs). Drugs that target various aspects of the Env-mediated entry process are also available.
Do we need new antiretroviral drugs?
Current cART is highly effective in suppressing HIV replication in infected individuals. However, current antiretroviral drugs do not eliminate the virus-infected cells from the body; these cells can persist in a resting state for decades. Current drugs thus must be administered for the lifetime of the infected individual, leading in some cases to toxicity and poor adherence.
A growing problem, particularly in resource-limited settings, is the development of drug resistance. Thus, in addition to improved drug regimens, better viral load monitoring to detect incomplete viral load suppression, and improved adherence, new classes of antiretroviral inhibitors will be needed moving forward for the long-term care of people living with HIV.
New targets for therapeutic intervention
Basic HIV research has revealed numerous new targets for antiretroviral drug therapy. These include two entry inhibitors that target Env. Two new classes of inhibitors target Gag:
1) Maturation inhibitors, which are under clinical development, block a late step in the PR-mediated processing of Gag thereby blocking virus maturation(1).
2) Lenacapavir, recently approved by the FDA, is a long-acting molecule that binds the capsid domain of Gag and disrupts HIV-1 infection at several steps.
Most notably, lenacapavir interferes with nuclear import and integration.
A recent study showed that injections of lenacapavir once every six months markedly reduce viral loads in infected individuals(2). Long-acting formulations are increasingly being considered for cART to avoid the need for daily oral dosing. In summary, continued investment in basic HIV research will be required to sustain the remarkable progress made in effectively treating people living with HIV.
References
- Kleinpeter, A. B., Freed, E. O. HIV-1 maturation: lessons learned from inhibitors. Viruses 2020: 12(9), 940.
- doi: 10.3390/v12090940
- Segal-Maurer, S., DeJusus, E., Stellbrink, H.-J., Castagna, A., Richmond, G. J., Sinclair, G. I., Siripassorn, K., Ruane, P. J., Berhe, M., Wang, H., Margot, N. A., Dvory-Sobol, H., Hyland, R. H., Brainard D. M., Rhee, M. S., Baeten, J. M. Molina, J.-M., CAPELLA Study Investigators. Capsid inhibition with Lenacapavir in multidrug-resistant HIV-1 infection. N. Engl. J. Med. 2022 386, 1793.
- doi: 10.1056/NEJMoa2115542

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


