Professor Giovanni Brandi (1,2) and Dr Simona Tavolari (1,2) from the University of Bologna discuss findings from preclinical and clinical studies that highlight the potential of hydrogen therapy in cancer treatment
Hydrogen is the lightest chemical element and is present in the atmosphere at non-toxic concentrations (less than one part per million) as a colourless and odourless gas. In humans, it is produced from carbohydrates not absorbed in the intestine through the anaerobic metabolism of some intestinal bacteria containing the enzyme hydrogenase. Then, it is partially diffused into the bloodstream and released from the lungs during exhalation.
Considered for a long time a physiologically inert gas, recent studies have demonstrated that hydrogen possesses significant antioxidant and anti-inflammatory properties that make the use of this molecule potentially applicable in many different clinical settings, including cancer treatment.
It is well known that, compared to normal cells, cancer cells show an altered redox environment, with a higher rate of reactive oxygen species (ROS) production, due to a different energy metabolism that produces ATP in mitochondria through aerobic glycolysis (Warburg effect) instead of oxidative phosphorylation.
ROS are highly unstable molecules that can easily react with carbohydrates, lipids, proteins and nucleic acids due to the presence of one or more unpaired electrons in their structure.
Since a tight regulation of intracellular ROS levels is essential for normal cell functions and survival, cells are endowed with endogenous antioxidant systems; however, when this cellular defence system is less efficient, oxidative stress occurs, favouring the acquisition of a malignant phenotype.
The pro-tumorigenic effects of ROS rely on different molecular mechanisms, including:
- Induction of acute/chronic inflammation through the production of pro-inflammatory cytokines.
- Induction of genomic instability through DNA single/double-strand breaks and the formation of 8-oxo-guanine, which determines G-T or G-A transversions.
- Activation of signalling transduction cascades involved in cell proliferation and survival.
- Promotion of tumour angiogenesis by inducing the secretion of vascular epithelial growth factor and angiopoietin.
- Increase of metalloproteinase expression that promotes cell migration and invasion.
- Induction of immunosuppression due to inhibition of CD8+ cytotoxic T lymphocytes proliferation and activation.
- Promotion of intestinal dysbiosis due to changes in the composition of the microbiota.
The higher ROS production in cancer cells compared to normal cells makes them more sensitive to changes in intracellular ROS levels, paving the way for using some antioxidant molecules as a possible anti-cancer strategy. However, large-scale clinical trials with traditional antioxidants such as vitamin C, vitamin E and β-carotene did not reach the expected results.
It has been hypothesized that this failure could be related to the inability of these molecules to enter the mitochondria and, therefore, effectively decrease intracellular ROS production. Conversely, hydrogen can quickly penetrate subcellular organelles such as mitochondria and react selectively with the hydroxyl radical, forming water without affecting normal cellular redox reactions.
Molecular mechanisms linked to hydrogen antitumoral effects
Currently, some preclinical and clinical studies support the use of hydrogen as a possible therapeutic approach for cancer treatment. The first study demonstrating the antitumor efficacy of hydrogen administration was published in 1975 by Dole et al., who reported a decrease in squamous cell carcinoma cell growth in mouse models treated with this gas.
Since then, the antitumor effect of hydrogen has been suggested in different tumour types and with different delivery modalities, including inhalation, drinking water, injection of hydrogen-saturated saline and bath in hydrogen-dissolved water.
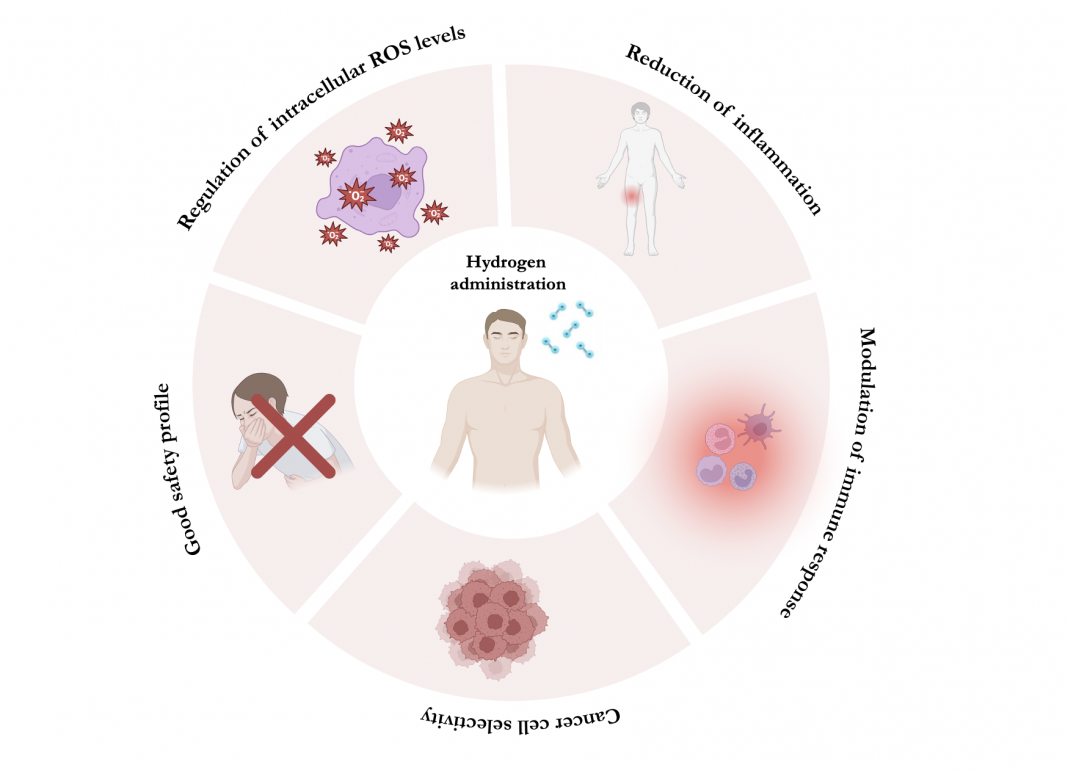
Overall, findings from these studies suggest that hydrogen antitumor effects are mediated by different mechanisms (Figure 1), including its ability to regulate intracellular ROS levels and the expression of some antioxidant enzymes, such as superoxide dismutase and glutathione.
Moreover, hydrogen administration has been shown to significantly decrease tissue damage caused by acute/chronic inflammation.
The reduction of inflammation not only relies on the antioxidant properties of this molecule (inflammation is, in fact, closely linked to oxidative stress), but also on its ability to inhibit the expression of pro-inflammatory cytokines (such as interleukin-1β, IL-6, IL-8, IL-10, tumour necrosis factor-alpha and interferon-gamma) and to modulate the activity of the immune system through the reduction of neutrophil and M1 macrophage infiltration and the restoration of Treg lymphocytes.
Hydrogen therapy for cancer treatment
Since 2017, the number of studies on hydrogen therapy and its potential applications in cancer treatment has steadily increased, indicating that this topic is gaining an increased interest in cancer research. Very importantly, in most of these studies, hydrogen administration demonstrated a certain selectivity towards cancer cells over normal tissues, along with a good safety profile.
Although, to date, there is no data on long-term toxicity, the occurrence of side effects related to hydrogen overdose is unlikely, due to its ability to selectively react with hydroxyl radicals forming water and, in parallel, to be eliminated by exhalation.
These physico-chemical properties differentiate hydrogen from other traditional antioxidant molecules, for which, in humans, the effective dose exceeds the maximum tolerated dose. Undoubtedly, current available studies are still immature to draw definitive conclusions on the role of hydrogen administration in cancer treatment; however, the encouraging results obtained in these pilot studies strongly encourage further clinical studies in different settings of cancer patients.
References
- Medical Oncology, IRCCS Azienda Ospedaliero- Universitaria of Bologna, Bologna, Italy;
- Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.