Professor Channakeshava S Umeshappa from Dalhousie University discusses the burden of primary biliary cholangitis and how understanding immune mechanisms may help to treat it
Primary biliary cholangitis: The most common autoimmune-mediated liver disease
Primary biliary cholangitis (PBC) is characterised by chronic inflammation of the interlobular bile ducts, leading to fibrosis, cirrhosis, and liver failure if left untreated.
Symptoms of PBC include fatigue, jaundice, itchy skin, abdominal pain, nausea or vomiting, dry eyes and mouth, and bone and joint aches.
The incidence of PBC is increasing worldwide,(1) but is most common in Europe and North America.(2) PBC has a female predominance, affecting primarily middle-aged women (over 90% of the PBC cases). Clinically, it is often diagnosed by detecting anti-mitochondrial autoantibodies and elevated alkaline phosphatase enzymes in blood tests.
Immunological mechanisms of primary biliary cholangitis
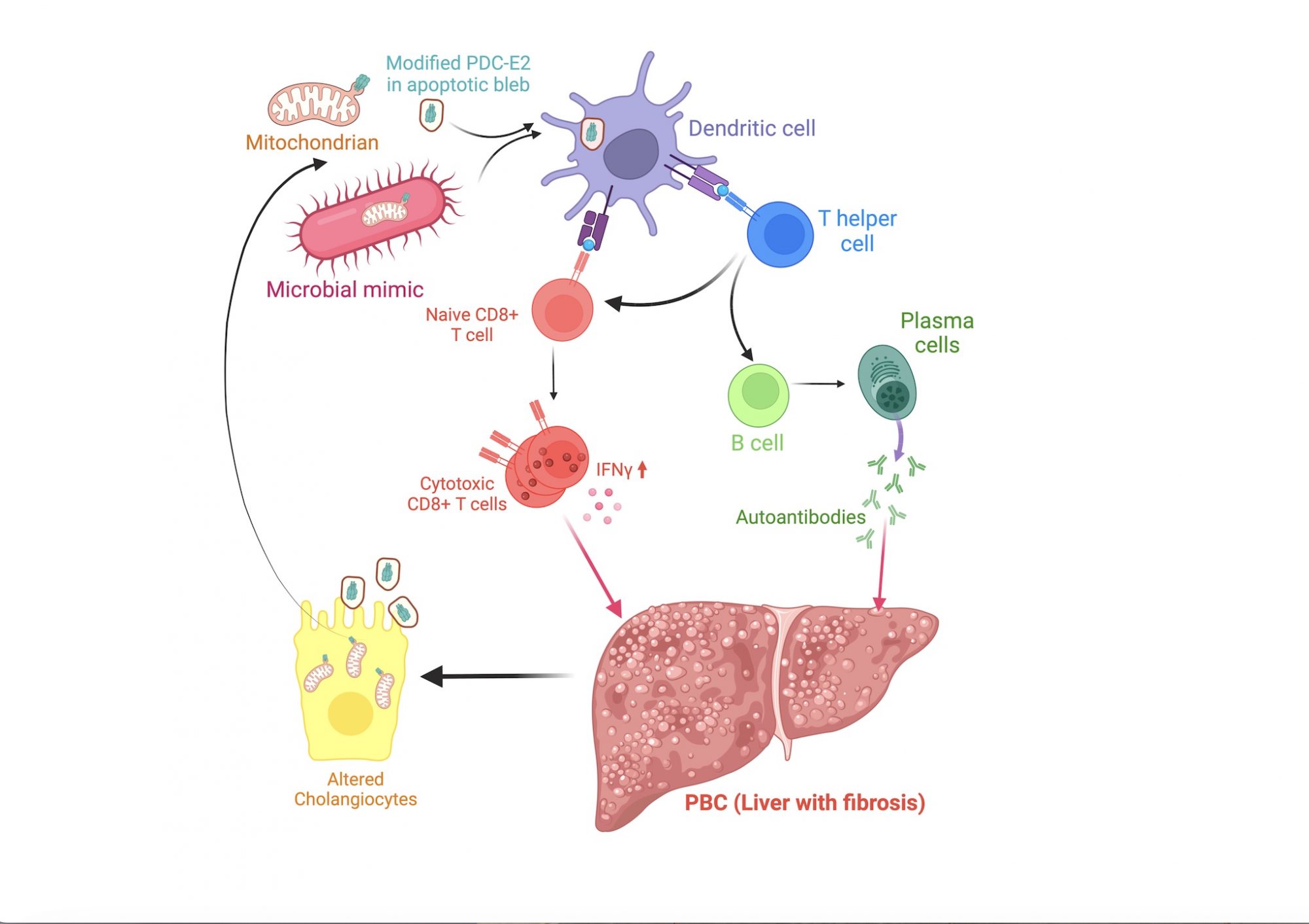
The exact cause of PBC is less understood. However, a combination of genetic risk and environmental factors, such as exposure to an environmental mimic of a modified E2 component of the pyruvate dehydrogenase complex (PDC-E2), is believed to predispose individuals to PBC development. (3)
These risk factors appear to cause loss of tolerance to PDC-E2 in biliary epithelial cells, leading to the destruction of these cells by autoreactive T and B cells, cirrhosis, and liver failure (Fig. 1).
Unfortunately, immunologists have not studied PBC until recently due to its ‘rare’ designation. We do not know what precisely causes tolerance breakdown to PDC-E2, leading to T and B immune cell-mediated liver destruction.
My previous postdoctoral work identified pathogenic and regulatory immune cells in the body that can promote or prevent PBC development, respectively.
Using PDC-E2-epitope-specific major histocompatibility complex-II-based nanomedicines (pMHC-NMs), we have expanded cognate (PDC-E2-epitope- specific) CD4+ T cells in two PBC mouse models having different genetic backgrounds.(4) The NMs likely targeted the pathogenic cells. This is because they displayed the epitope that overlaps with a repetitive element in PDC-E2 with homology to the E. coli bacterial antigen – playing a role in PBC development. (5)
As these pathogenic CD4+ T cells are known to promote autoreactive responses of both CD8+ cytotoxic T lymphocyte and B cell responses, they likely play crucial roles in autoimmunity development. In the subsequent studies, we also found increased levels of proinflammatory type 1 invariant Natural Killer T cells (iNKT1) in the liver of mice with established PBC.
Aditionally, we discovered the presence of a liver-specific regulatory iNKT subset in both mice and humans that may have a role in the control of PBC development.(6) However, these inflammatory and regulatory T cells’ contributions to PBC development, their nature, and the mechanisms by which they cause or regulate PBC are unknown. Hence, my lab is currently investigating the potential mechanisms used by these immune cells in the regulation of PBC development.
This sheds light on identifying novel therapeutic target to block the destructive autoinflammatory responses and the development of next-generation therapies.
Primary biliary cholangitis and the future of treatments
There is no curative treatment for PBC. Hence, current treatment centres on preventing complications and alleviating symptoms.
Non-specific ursodeoxycholic acid and obeticholic acid are indicated, but they are poorly effective in 30-40% of the patients, who will continue to have disease progression.(7) Therefore, there is an urgent need to develop disease-specific treatments for PBC.
Since PBC results from autoimmune-mediated damage, targeting the key immune cells and pathways involved in the disease represents an ideal therapeutic approach.
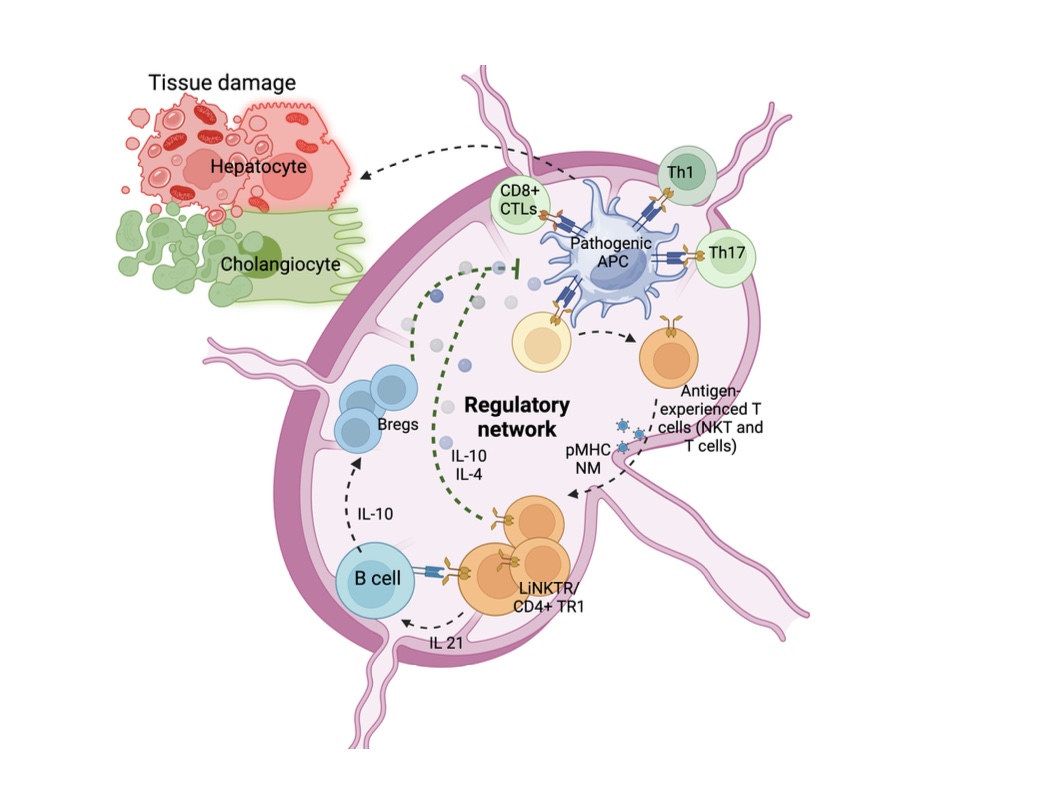
In my previous studies, using PDC-E2- relevant pMHCII-NMs, we programmed and expanded PDC-E2-epitope-specific CD4+ T cells into regulatory cells (4,8) (Fig. 2). These cells established local regulatory network by favouring regulatory B cells and suppressor neutrophil formation and suppressing pathogenic antigen-presenting cells and controlled PBC in mouse models.
Furthermore, results showed autoimmune-hepatitis (AIH)-relevant pMHCII-NMs can also expand AIH- epitope-specific CD4+T cells and control primary biliary cholangitis in a liver-centric manner, irrespective of autoantigen specificity.
In a more recent study, we expanded liver-specific regulatory iNKT cells and controlled PBC.(6) Undoubtedly, these NMs are promising candidates for human PBC therapy. However, due to the chronic nature of PBC and the relatively short-term effects of these drugs, the patients need to be treated for life, which may result in unwanted side effects.
Encouraged by the success of these drugs, my laboratory is currently focusing on developing newer and better mechanism-based immunotherapies that provide long-term protection against PBC in a radical manner.
References
- Galoosian et al., J Clin Transl Hepatol 8, 49-60 (2020).
- Tanaka, A. Clin Mol Hepatol 27, 1-21 (2021).
- Gulamhusein & Hirschfield. Nat Rev Gastroenterol Hepatol 17, 93-110 (2020).
- Umeshappa et al. Nat Commun 10, 2150 (2019).
- Van de Water et al., Hepatology 33, 771-775 (2001).
- Umeshappa et al. Nat Commun 13, 3279 (2022).
- Corpechot et al., JHEP Rep 1, 203-213 (2019).
- Umeshappa et al., Cell Rep 34, 108919 (2021).

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.