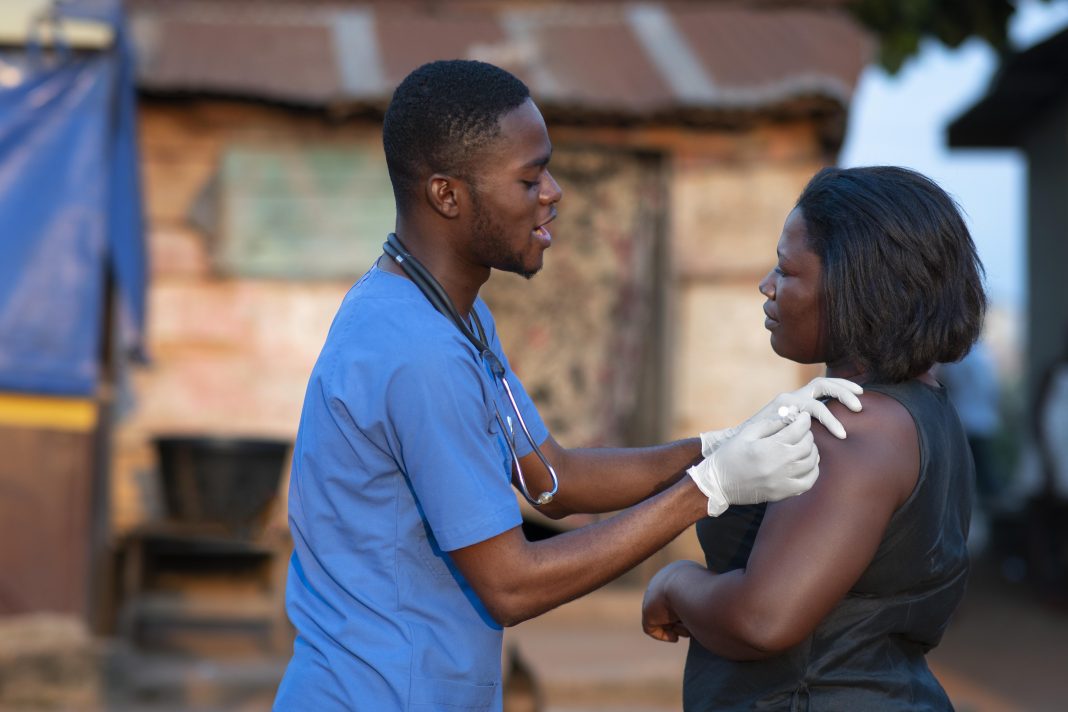
Unither Pharmaceuticals is dedicated to innovating and expanding capacity to provide equitable vaccine accessibility in developing countries and enhance preparedness for future pandemics. Here, they explain how the Euroject® device can support their goals
The SARS-CoV-2 pandemic has had a devastating impact worldwide, causing seven million deaths and infecting more than 775 million people. (1) Despite the rapid development and approval of vaccines, their distribution faced significant hurdles due to shortages of consumables like glass vials. To prepare for ‘Disease X’, a term coined by governments to describe an unknown pathogen that could emerge and cause an epidemic or pandemic, many steps have been taken to increase preparedness. In this context, Blow-Fill-Seal (BFS) technology has emerged as a game-changing solution, particularly for developing countries, owing to its efficient and scalable packaging design.
The evolution of BFS technology
BFS technology originated from a 1963 patent. Initially, it was used for packaging food, cosmetics, and medical devices in non-sterile conditions. (2) The first bottle pack (BP) machine was launched in 1964. By the early 1970s, the pharmaceutical industry adopted
it for large-scale packing of pharmaceutical solutions. (3) By the 1980s and 1990s, BFS was established for sterile solutions, evolving into a preferred method for unit-dose packaging.
Today, BFS technology is recognized in the 2022, EU GMP Annex 1: Manufacture of Sterile Medicinal Products. Its efficiency is notable, with machines capable of producing 200-300 million vials annually per machine.

Revolutionizing vaccine distribution with BFS technology
Unither Pharmaceuticals, a worldwide leader in BFS technology with 30 years of experience, introduced Euroject® in September 2021. Euroject® is a disruptive injection device combining a BFS unit-dose vial with a needle hub for the administration of vaccines, biologics, and chemical API through subcutaneous, intramuscular, and intradermal routes of injection.
The Euroject® project is supported by a dedicated BSL2 (Biosafety Level 2) plant in Amiens, France, which aims to fill one billion doses annually by 2027.
BFS: Ideal for vaccine packaging
The BFS process is a fully automated, aseptic technology that involves melting plastic polymer granules at high temperatures, extruding them, and blowing them into vial shapes. Despite the high temperatures, the process is rapid and has been optimized, minimizing exposure time and preserving the integrity of temperature-sensitive molecules like biologics and vaccines.
Previously, the use of BFS vials for vaccines has proven their efficacy and stability. For instance, MedImmune’s Phase III clinical study in the early 2010s demonstrated that BFS-packaged influenza vaccines were immunogenically non-inferior to traditional glass vial packaging. (4) Similarly, GSK’s Rotarix® vaccine, used in low- and middle-income countries, showed comparable stability in BFS vials. (5)
Optimizing BFS for global vaccine accessibility
Euroject® is designed to address the challenges of vaccine distribution in developing countries. The BFS technology ensures a cost-effective and scalable solution, reducing dependency on glass vials and mitigating the risk of cross-contamination. Unither’s rigorous testing, including the use of surrogate vaccines like the SARS-CoV-2 RBD antigen, confirms the compatibility of BFS with biologics and vaccines. (6)
Scientific validation of BFS technology
Recent studies and trials have further validated the effectiveness of BFS technology for vaccine packaging:
- Vaccine delivery efficiency:
- Tests conducted demonstrated that the Euroject® system delivers 0.5ml of vaccine with a minimal loss compliant with ISO standard 7889 and the Target Product Profile from PATH, ensuring the entire dose reaches the patient.
- Integrity and safety:
- Performance studies showed 100% sealing integrity of the ampoule and no leakage or fragmentation upon opening, complying with ISO standard 80369. This ensures the vaccine remains sterile and uncontaminated throughout the delivery process. Furthermore, due to the absence of syringes, Euroject® eliminates contamination due to syringe reuse, and the needles can also be fitted with safety caps to prevent reuse.
- Compatibility with sensitive molecules:
- Unither has developed methods to adapt the BFS process against detrimental operating conditions such as temperature, light, and oxygen.
Advantages of Euroject® for vaccine accessibility in developing countries
- Cost-effective production:
- BFS technology increases vaccine affordability for developing countries by reducing raw material costs, as the material is five times less expensive than those used in traditional glass vials.
- Scalability and efficiency:
- BFS production lines can operate at two to three times the capacity of prefilled syringes (PFS) lines, ensuring a rapid and scalable response to vaccination needs with a very limited footprint.
- Supply chain resilience:
- Utilizing a single raw material, low-density polyethylene (LDPE), BFS not only reduces the risk of supply chain disruptions, ensures a consistent supply of vaccine containers and eliminates the need for multiple components such as glass, rubber, and aluminum but also reduces breakage.
- User-friendly design:
- Healthcare professionals (HCPs) have highlighted the simplicity and reliability of using Euroject®. The simplicity of this device could allow the administration during vaccination campaigns, usually done by HCPs, to other groups such as police officers, firefighters, and health students, allowing HCPs to concentrate on more specialized tasks.
- Environmental sustainability:
- The BFS process is more sustainable than traditional methods due to its lower environmental impact and efficient use of resources, including energy and recyclability of LDPE, as it is a mono-material and has the lowest packaging mass. These factors align with global sustainability goals to reduce the ecological footprint of vaccination programs.
Field-tested and proven
Studies have shown that the Euroject® system maintains the integrity and efficacy of vaccines with no significant impact on the delivered volume or quality of the vaccine, regardless of needle size or injection conditions.
A vision for global health security
The new Euroject® facility in Amiens set to open for routine (or commercial) production in late 2027 has a production capacity of one billion doses per year. Euroject® offers an alternative solution for the global distribution of vaccines and biologics. Along with Unither’s global presence (sites in France, the US, Brazil, and China), the site in Amiens will play a pivotal role in meeting global vaccination needs.
Unither Pharmaceuticals’ commitment to innovation and capacity expansion will significantly contribute to ensuring equitable vaccine accessibility in developing countries and improving preparedness for future pandemics.
References
- https://ourworldindata.org/covid-cases
- GERHARD, H. (1963). Germany Patent No. 1272807
- Oschmann, R., & Schubert, O. E. (1999). Blow-Fill-Seal Technology, vol. 40,. CRC Press Inc
- Eric, A. S., Robert, J., Joseph, A. S., Supoat, C. M., Filip, D., & Mallory, R. M. (2012, October 14). Immunogenicity of a quadrivalent Ann Arbor strain live attenuated influenza vaccine delivered using a blow-fill-seal device in adults: a randomized, active-control. Influenza Journal, pp. 1142-1150
- Manjari, L., Courtney, J., Changcheng, Z., Nancy, A. H., Chris, L. M., David, M. K., Dexiang, C. (2016, March 29). Stability of live attenuated rotavirus vaccine with selected preservatives and primary containers. Vaxine, pp. 2483-2489
6. Fontayne, A. (2022, December 13 ). Blow-fill-seal technology and vaccine delivery. European Biotechnology. Retrieved from European Biotechnology

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.