Helen Greenwood Hansma, from the University of California at Santa Barbara, questions if there is an elephant in the room of research when it comes to the origins of life
Shouldn’t researchers on the origins of life look in environments with high potassium (K)?
All types of living things have many times more potassium ions (K) than sodium ions (Na) in their cells (Figure 1). Don’t you think life must have started in a place with more K than Na, like the spaces between mica sheets? (1-5) Sea and land water have less K and more Na. Why are researchers focusing on these high-Na environments? Is this an elephant in the room of research on life’s origins? (Figure 2) (There is a pump in the cell membranes now to keep all this K in our cells, which are in close contact with the Na-rich blood. It takes a lot of energy to run this pump, called the Na/K ATPase.)
Mica is a clay-like mineral with K between its sheets, holding them together (Figure 3A). The mineral sheets in most clays are held together by Na, and these clays swell and shrink when they get wet and dry. These cycles of swelling and shrinking happen because Na is small, and there is room for a few molecules of water between the clay sheets, even when the clay is dry. K is a bit bigger, so there is no water between the sheets of mica when they are dry. Wouldn’t life start more easily in an incubator that wasn’t shrinking and swelling whenever it was drier or wetter?

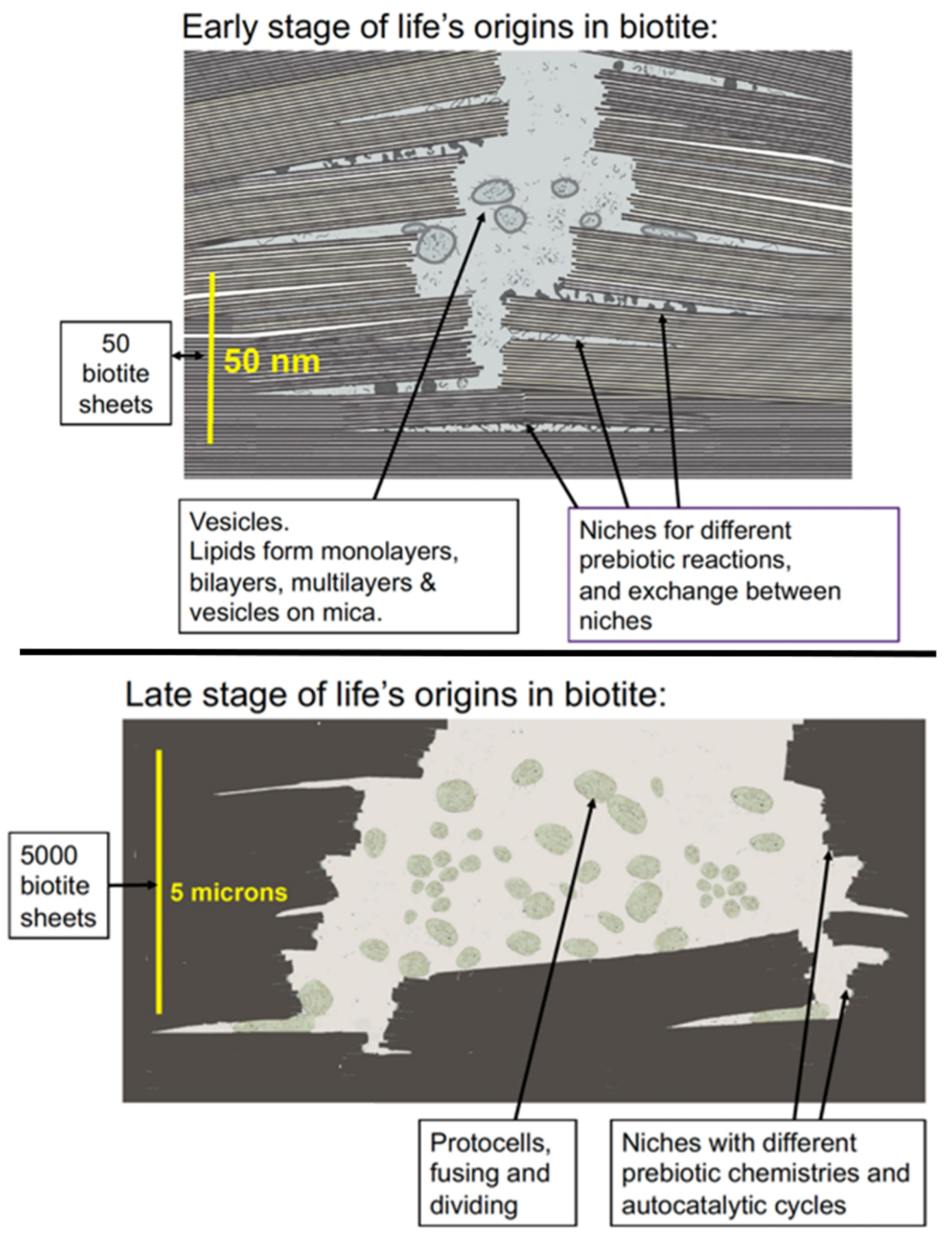
Figures 3-5 show the possible origin of life in black mica, biotite, because black mica is rich in iron, which may be the only mineral required for life’s origins, according to recent research. (6) Mica sheets have other advantages as an incubator for life’s origins. The edges of the sheets move, open, and shut due to water flow between the sheets or to temperature changes. These moving mica sheets have mechanical energy (5), an important energy source even in living organisms. (7)
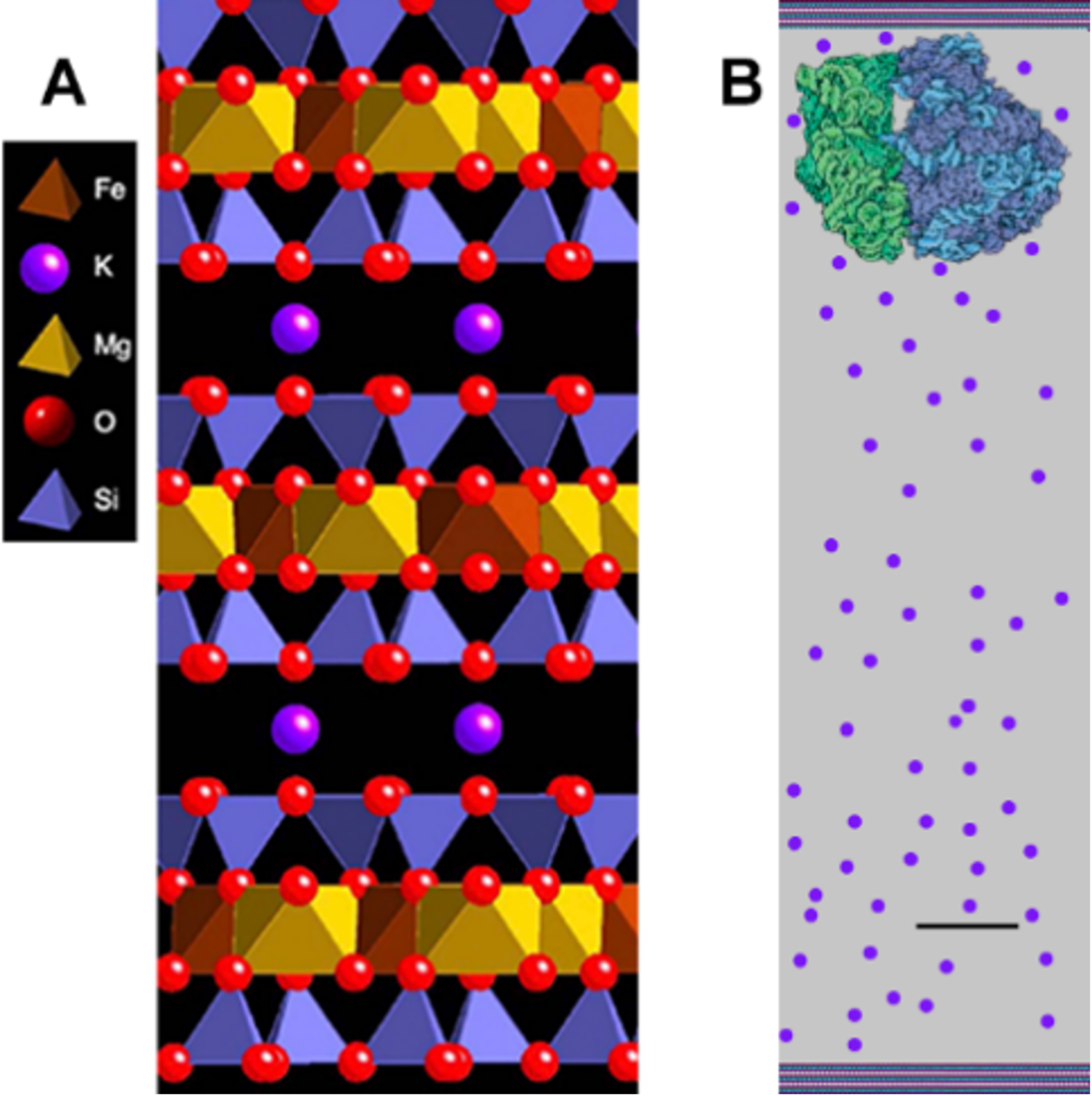
The environment between mica sheets also has many other similarities to living cells, further supporting the possibility that mica was the substrate on and within which life emerged. (2) Amino acids and nucleotides can polymerize to form proteins and DNA by drying, but the polymers hydrolyze and break down in the presence of water. In mica, wet-dry cycles occur only at the split edges of mica sheets and the rest of the space between the mica sheets is not wetting and drying.

These slow wet-dry cycles at the edges of the mica sheets result in longer polymers during the longer drying cycles before hydrolysis occurs during the wet phase. These longer polymers stick to mica better than short polymers and are, as a result, more likely to remain bound to the surface, making it more likely that these molecules will continue to evolve until there are living cells. Atomic force microscopy (AFM) shows that long DNA molecules bind better to mica than short DNA molecules. (8)
References:
- Hansma, H G (2010) “Possible origin of life between mica sheets.” Journal of Theoretical Biology 266(1): 175-188.
- Hansma, H G (2022) “Potassium at the Origins of Life: Did Biology Emerge from Biotite in Micaceous Clay?” Life 12(2), 301; https://doi.org/10.3390/life12020301
- Hansma, H G “When and where did intracellular potassium arrive at the origins of life?”. Biophysical Society Past Networking Events 2021. https://www.biophysics.org/past-networking-events/when-and-where-did-intracellular-potassium-arrive-at-the-origins-of-life
- Hansma, H G (2022). “DNA and the origins of life in micaceous clay.” Biophysical Journal 121(24): 4867-4873.
- Hansma, H. G., (2024) “Mechanical energy at the origins of life”, Open Access Government October 2024, pp.394 https://www.openaccessgovernment.org/article/mechanical-energy-at-the-origins-of-life/183443/
- Jena E. Johnson, Theodore M. Present, Joan Selverstone Valentine. (2024) “Iron: Life’s primeval tran-sition metal”, Proceedings of the National Academy of Sciences, 121 (38) DOI: 10.1073/ pnas.2318692121
- Biophysical Journal, for example, is preparing a Special Issue on “Materials and Measurement in Mechanobiology”
- Hansma, H., I. Revenko, K. Kim and D. Laney (1996). “Atomic force microscopy of long and short double-stranded, single-stranded and triple-stranded nucleic acids.” Nucl. Acids Res. 24(4): 713-720.
Author Email: hhansma@ucsb.edu
Author Website: https://web.physics.ucsb.edu/~hhansma/mica.htm

![Fig 1 Figure 1: Ratios of the concentrations of Sodium ions [Na+] and Potassium ions [K+] in water on land and in the sea vs in living cells and in blood.](https://www.openaccessgovernment.org/wp-content/uploads/2024/12/Fig-1-1068x968.png)
