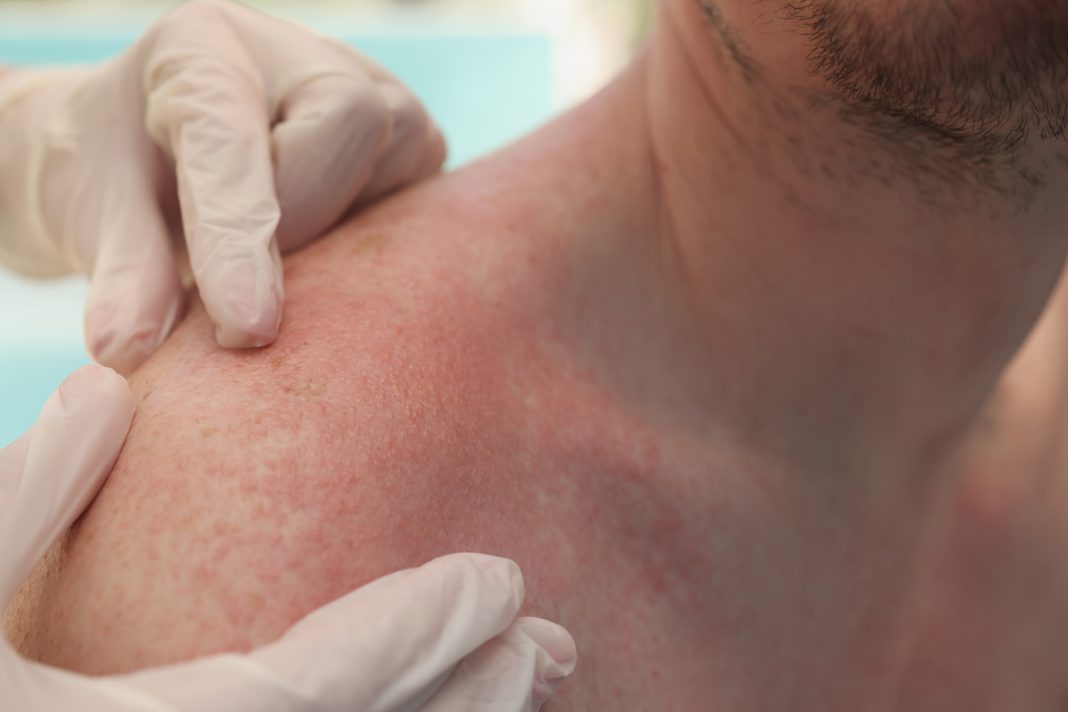
Kirstine Juhl Belongie discusses the impact, symptoms, and possible treatments related to EPP and XLP, two debilitating disorders that cause extreme pain following exposure to sunlight
For most people, a warm sunny day feels comfortable on the skin, but sun rays can produce excruciating pain for a rare group of people born with a genetic mutation in two specific genes. Photosensitivity or even phototoxicity occurs for many reasons (genetic, drug-induced, or allergic reaction from an unknown cause) and can be expressed as rashes, blistering, redness, or pain.
Protoporphyria – a rare genetic condition
Erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLP) are two debilitating disorders that cause extreme pain from phototoxic attacks following sunlight exposure to visible light. (1) The disorders are extremely rare, with estimations that one in 75,000 to 200,000 people are born with inherited genes that affect how the body makes heme.
Causes of EPP and XLP
Heme, part of hemoglobin, is made by the intricate coordination of eight enzymes, and it is the flow through this pathway that is disturbed in porphyria. In EPP, the last enzyme (ferochelatase) is not able to add iron and thereby finish all the precursors to heme. In XLP, the first enzyme (aminolevulinate synthase) has increased activity, leading to increased precursors throughout the pathway. Both genetic conditions lead to the buildup of metal-free protoporphyrin, the last precursor before heme, within erythrocytes. Protoporphyrin also accumulates in plasma, liver, and skin vasculature. These highly increased levels of metal-free protoporphyrin lead to people experiencing debilitating skin reactions when exposed to sunlight. The excruciating pain associated with the skin reactions can significantly affect their quality of life.
Symptoms of EPP and XLP
Symptoms vary from person to person and can accumulate during the day depending on the amount of sunlight a person is exposed to. Symptoms can occur in some within as little as a few seconds of sunlight exposure, whereas others can stay in sunlight for a few hours. About a quarter of the patients feel the symptoms come on after just ten minutes of sunlight, and roughly 60% after 30 minutes. The first symptoms are described as tingling, itching, stinging, and hotness, and if the person seeks shade or goes inside, the symptoms typically subside. However, indirect and reflected light off water or buildings often catches people by surprise, and it can be hard to avoid. Spending the next two to seven days after the sun-mediated injury is needed for full recovery in absolute darkness and excruciating pain.
In addition to pain, cutaneous symptoms include edema, erythema, and occasional blistering. Patients are advised to strongly avoid sunlight, but even without sunlight, excessive protoporphyrin can seriously injure internal organs. About one-third of patients experience liver disease when insoluble protoporphyrin accumulates and can crystalize to form gallstones.
Treatments for EPP or XLP
Patients are advised to avoid sunlight by wearing protective clothing and opaque sunscreen and staying indoors during sunlight hours. No therapies can alleviate the pain from the skin reaction once it has started.
Currently, no treatment corrects the genetic defects in EPP or XLP, nor are there any treatments that normalize the protoporphyrin levels. A bone marrow transplant is the only way to fully eliminate the culprit. The only approved treatment in the United States, the European Union, and Australia is afamelanotide, an implant subcutaneously administered every two months to increase the tolerance to sunlight in adults with EPP.
Children and quality of life
Currently, there are no medications approved for children or adolescents with EPP or XLP.
EPP and XLP usually manifest in early childhood after a short exposure to sunlight. Sometimes, the skin manifestations are subtle and make the source of the pain experienced invisible, leading to long diagnostic delays of more than ten years after the onset of symptoms. Often, numerous providers (pediatricians, allergists/allergologists, hematologists, dermatologists, and hepatologists) are consulted before a diagnosis is confirmed with either a protoporphyrin blood test or genetic testing. There remains an unmet need for effective treatments to increase time in the sun and, thus, increase the quality of life for pediatric patients living with EPP and XLP.
Investigative research into dersimelagon
Clinical studies with adults and adolescents living with EPP and XLP are underway with a new investigational drug called dersimelagon. (2) Dersimelagon is designed to induce pigmentation (eumelanin production) in the skin without the need for UV, phototherapy, or sunlight exposure. This creates a protective barrier against sunlight penetrating the skin, potentially increasing time out in the sun and reducing the severity of painful reactions in patients with EPP and XLP.
The first clinical study with dersimelagon in patients with EPP and XLP was a phase 2 study done at nine locations in the US. (3) During 16 weeks of treatment, patients received different doses of dersimelagon or a placebo treatment, and their behavior and symptoms were self-recorded.
The patients were encouraged to seek sunlight exposure until the first symptoms appeared, the very thing they were afraid of. Evaluations were conducted to determine the difference in the amount of time patients could spend in sunlight without symptoms or if their symptoms were less severe than those treated with a placebo. Patients were carefully and thoroughly followed at expert clinics to understand how dersimelagon affects the patient’s quality of life and clinical measures.
This phase 2 study included 102 adult patients with EPP and XLP and showed promising efficacy results in the patient’s ability to be exposed to more time in sunlight before experiencing symptoms, their number of pain events was reduced, and quality of life measurements improved. The safety profile was tolerable, with most adverse events being mild or moderate in severity. Results from the trial support the effectiveness and safety of dersimelagon and its further development as a potential oral treatment option for increasing light tolerance in patients with erythropoietic protoporphyria or X-linked protoporphyria.
The potential for dersimelagon to be a therapeutic treatment for people living with photodermatoses such as EPP and XLP is being tested in a new large-scale phase 3 global clinical study. The study is currently underway to potentially offer additional treatment options for adults and adolescents living with EPP and XLP. (4)
Acknowledgment
Thanks to patients, advocates, (5) clinical experts, and personnel for all their dedication and hard work.
References
- Dickey, A, Naik, H, Keel, S. Evidence-based consensus guidelines for the diagnosis and management of erythropoietic protoporphyria and X-linked protoporphyria J Am Acad Dermatol 2023;89:1227-37.)
- Suzuki, T, Kawano, Y, Matsumoto, et al., (2022) Melanogenic effect of dersimelagon (MT-7117), a novel oral melanocortin one receptor agonist. Skin Health Dis, 2(1), e78. https://www.nejm.org/doi.org/10.1002/ski2.78
- Balwani, M, Bonkovsky, HL, Levy, et al., (2023) Dersimelagon in erythropoietic protoporphyrias. New Engl J Med, 388(15), 1376–1385. https://www.nejm.org/doi/10.1056/NEJMoa2208754
- ClinicalTrials.GOV study: NCT06144840
- Patient advocacy groups: https://www.porphyria.org/ and https://porphyriafoundation.org/