Pharma researchers Uwe Grether and Julie Blaising from F. Hoffmann-La Roche Ltd. highlight the vast therapeutic potential of MAGL inhibition for central and peripheral diseases
The endocannabinoid system (ECS) is an important lipid signalling system that is highly conserved among vertebrates. It plays a key role in many human health and disease states.(1) Key components of the ECS are the endocannabinoids (eCBs), in particular 2-arachidonylglycerol (2-AG) and N-arachidonoylethanolamine (AEA).
The eCBs bind to and activate the type-1 and type-2 cannabinoid receptors (CB1R and CB2R, respectively), which are highly important targets for treating multiple human diseases ranging from metabolic indications to neurodegenerative disorders.(2)
Metabolic enzymes and transporters are involved in the control of eCB lifespan and, hence, biological activity. The most important of these proteins is the serine hydrolase monoacylglycerol lipase (MAGL),(3) an enzyme that terminates the most abundant endocannabinoid transmitter 2-AG and thus holds great potential for the treatment of multiple disorders, including inflammation, neurodegeneration, and cancer.(4)
Where is MAGL located?
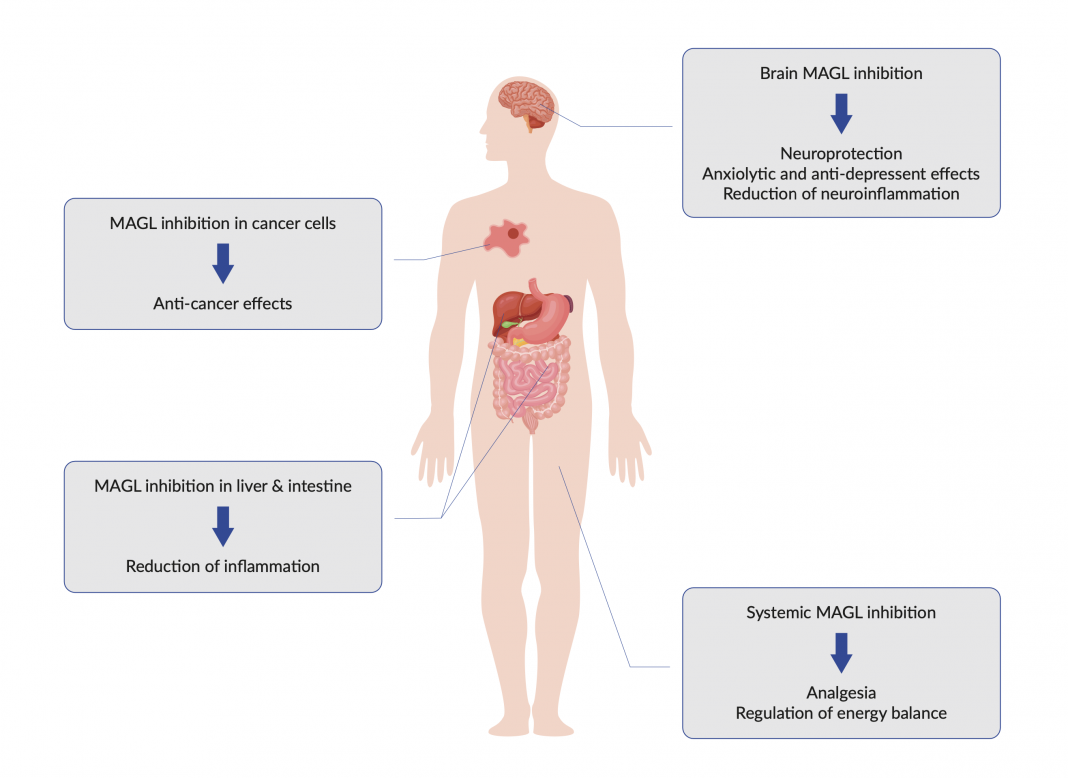
MAGL is expressed in specific areas of the brain that match the expression of CB1R such as the hippocampus, cerebellum, and cortex. It is the main enzyme to terminate the CB1R- mediated effects of endogenous 2-AG. Furthermore, MAGL-mediated degradation of 2-AG to glycerol and arachidonic acid (AA) sustains the supply of arachidonate precursor for cyclo-oxygenase (COX) mediated biosynthesis of inflammation-mediating prostaglandins and eicosanoids.. Outside the central nervous system (CNS), MAGL is widely distributed throughout the body, where it plays an important role in modulating both CB1/2R-dependent and independent processes that are of relevance for multiple pathophysiological conditions including, inflammation and cancer.
Therapeutic potential of MAGL inhibition
The blockade of MAGL has been shown to produce analgesic effects in animal models of inflammatory and neuropathic pain.(2a) Furthermore, there are beneficial effects on neuroprotection, neuroreparation, remyelination, control of stress-coping behaviour, mood, emotion, and substance-use disorders, and in mechanisms governing cancer progression and invasiveness and in mechanisms that regulate energy balance. In sum, MAGL inhibition has emerged as a promising target for various pathological conditions (Fig. 1).
Ligands targeting MAGL
MAGL contains a catalytic triad typical of serine hydrolases, composed of Ser122, His269 and Asp239.(3) The protein’s 3D shape consists of seven parallel and one antiparallel strand surrounded by α-helices, and a more variable region, known as the lid domain, situated above the catalytic site, which shapes and protects the access of substrates to the active site. Most of the synthetic MAGL inhibitors target the active site of the enzyme.(5) Some contain reactive groups e.g. carbamates or ureas that form a covalent bond with catalytic Ser122 and are termed covalent inhibitors. Reversible inhibitors interact with the enzyme through weak polar and hydrophobic interactions.
Both types occupy the active site access channel and representatives of each class have progressed into clinical development up to phase 2 trials. These ligands are structurally very diverse and clinical studies focus on neurological disorders, pain, multiple sclerosis, fibromyalgia, Tourette’s syndrome, epilepsy, post-traumatic stress disorder, cerebral hemorrhage, and inflammation.
Conclusions and future perspectives of MAGL-based medicines
Since the discovery of MAGL two decades ago many research groups have uncovered the critical role of terminating 2-AG-mediated signaling in the CNS and in peripheral tissues and the resulting vast therapeutic potential of this pharmacological target. Consequently, a multitude of ligands that block MAGL activity have been generated. Several of those are under clinical development aiming for the treatment of a variety of indications, many of them with an inflammatory and neurodegenerative background and will help to unravel the full therapeutic potential of this promising target within the next few years.
Acknowledgements
We wish to thank all colleagues who have contributed over the years to our investigations on MAGL.
References
- Maccarrone M, Di Marzo V, Gertsch J et al (2023) Goods and bads of endocannabinoid system as a therapeutic target:Lessons learned after 30 years. Pharmacol Rev. Epub ahead of print, doi: 10.1124/pharmrev.122.000600.
- a) New tools to interrogate endocannabinoid signaling. From natural compounds to synthetic drugs (Maccarrone M ed) pp 89-167, Royal Society of Chemistry, Cambridge; b) Grether U, Nazaré M (2023) CB2R agonists in the clinics: A treasure chest for treating inflammatory diseases. Open Access Government, https://www.openaccessgovernment.org/article/cb2r-agonists-clinics-treating-inflammatory-diseases/163225/.
- Karlsson M, Contreras JA, Hellman U et al (1997) cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 272(43):27218-23. 4. Dinh TP, Carpenter D, Leslie FM et al (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 99(16):10819-24. 5. Bononi G, Poli G, Rizzolio F et al (2021) An updated patent review of monoacylglycerol lipase (MAGL) inhibitors (2018-present). Expert Opin Ther Pat. 31(2):153-168.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.