Did mechanical energy power life’s origins before chemical energy such as ATP was available?
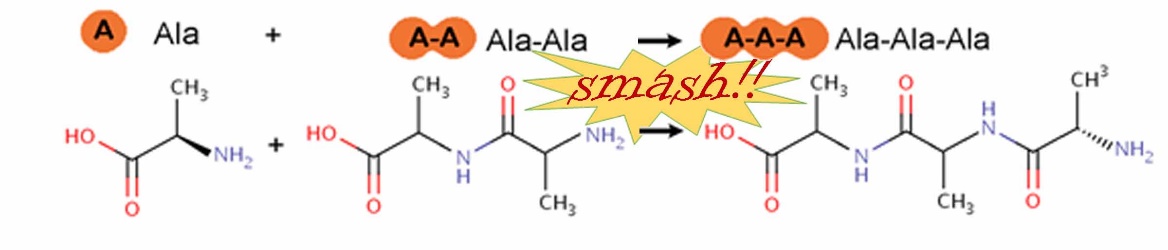
Mechanobiology is a hot topic in science today, with special issues on the subject in scientific journals. Was mechanical energy a way to form chemical bonds before the origin of life made mechanobiology possible? This makes a lot of sense, because life was not around yet to synthesize ATP (adenosine triphosphate), the chemical energy molecule that powers many of the processes of life. With mechanochemistry, molecules are simply smashed together to form covalent bonds between them (Figure 1).
Between mica sheets – a good place for mechanical energy to power the origins of life
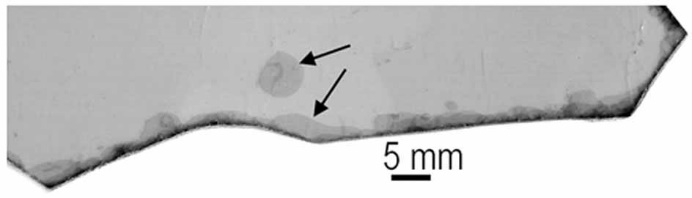
Mica sheets provide mechanical energy at their split edges and bubbles between the sheets (1-3), as shown in this photograph of a piece of mica (Figure 2) and the diagram of mica in Figure 3.
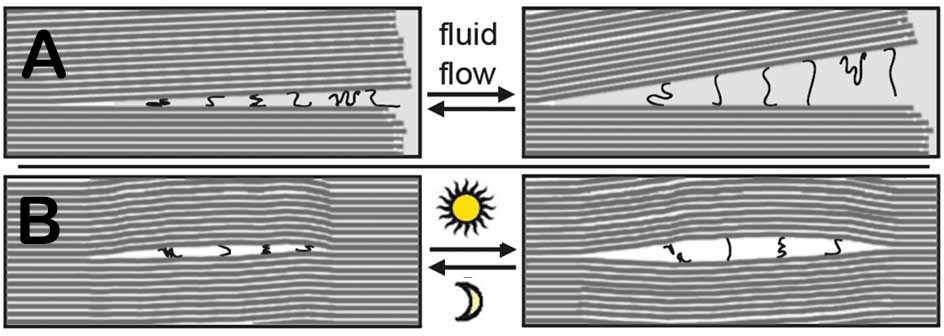
Water moving in and out between the mica sheets will push the sheets open and shut (Fig. 3a), while cycles of heat and cold will cause bubbles between the sheets to expand and contract (Fig.3b). This movement creates mechanical energy. Mechanical energy can be used for polymer rearrangement, as diagrammed in Fig 3, and covalent bond formation, as diagrammed in Fig. 1. Mechanical energy can also be used to bleb off vesicles and protocells in a prebiotic form of cell division (Fig. 4).
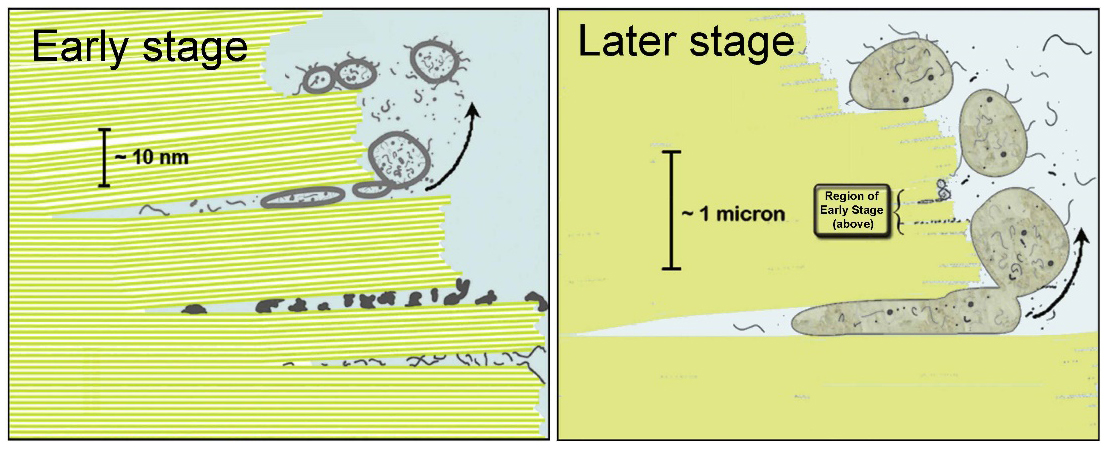
How might life have started between the sheets of mica?
In the Early stage (Fig. 4 left), individual ~1nm (nanometer) sheets of mica are seen. In the Later stage (Fig. 4 right), larger prebiotic structures have developed, and the individual mica sheets are not visible. Different prebiotic chemistries occur in different niches (spaces) between mica sheets. These niches provide partially enclosed spaces for the molecular evolution of the different structures that are essential for life’s processes. Lipid- coated vesicles form in the Early phase and encapsulate the molecular structures that form between the mica sheets. The vesicles fuse to form protocells in the Later phase.
Mechanochemistry is typically accomplished by ball milling or grinding in a mortar and pestle today. Therefore, mechanical energy at the origin of life might have been less elegant than what is possible with mica sheets.
Potassium is a neglected ingredient in almost all research about the origins of life
Why is the sodium/potassium [Na/K] ratio so low in living cells when the Na/K ratio is high in seawater and land water? If life started in water on land or in the sea, would early life have built an energy-intensive pump in its cell membranes to produce high intracellular concentrations of potassium ions [K+]?
Life likely started in an environment with high [K+], such as the environment between mica sheets.
References
- HG Hansma, Possible Origin of Life between Mica Sheets. J Theor Biol 2010. 266:175.
- HG Hansma, Mechanical Energy before Chemical Energy at the Origins of Life? Sci, 2020. 2:88.
- HG Hansma, DNA and the origins of life in micaceous clay, Biophys J 2022. 121:4867
Author Email: hhansma@ucsb.edu
Author Website: https://web.physics.ucsb.edu/~hhansma/mica.htm

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.