Electroactive microbes exchange electrons with their environment for survival
Most life forms use oxidation-reduction reactions for energy generation. At the basis of these reactions lies the exchange of electrons between two chemical species. Microbes access the necessary electrons for their metabolic processes from their environment through indirect or direct electron transfer pathways. While most microbes use soluble electron donors or acceptors, some have evolved the capability to also use insoluble solid materials for exchanging electrons through a process called extracellular electron transfer or EET.
These interactions are bidirectional and can be further classified based on the direction of electron flow. When bacteria reduce insoluble substances through respiration (i.e., electrons flow from the bacteria to the solid substance) the process is called reductive extracellular electron transfer or rEET. The opposite process where the insoluble substances are oxidized (i.e., electrons flow from the solid substance to the bacteria) is termed extracellular electron uptake or EEU.
EEU is a relatively newly discovered process in microbiology. The Bose research group is bringing an interdisciplinary approach to understanding the molecular mechanisms behind EEU and how it can be engineered to provide potential solutions to the climate crisis. These solutions come from the capacity of some EEU-capable microbes to use chemical energy (chemoautotrophs) or light energy (photoautotrophs) in a process like photosynthesis to convert carbon dioxide (CO2) into biomass. EEU-capable chemoautotrophs and photoautotrophs are quite common in nature and could not only be used to remove CO2 from the environment but also to concurrently produce bioplastics or biofuels through microbial electrosynthesis.
From natural minerals to bioelectrochemical reactors
Studying electroactive microbes and specifically EEU capable ones inside the laboratory requires methods for providing an electron source to the microbial cells while also measuring the electrical current flow through the system. This is most often achieved using macro- (or large-) scale electrochemical reactors where poised electrodes act as electron donors and are maintained using potentiostats at various potential ranges that match the potentials of materials oxidized by microbes in their natural habitat. The reactors are called bioelectrochemical systems or BESs, as the oxidation-reduction reactions involved in the microbial metabolism represent a part of the larger electrochemical system.
To achieve a better understanding of EEU capable microbes we must also understand this larger electrochemical system. BESs pose a difficult problem due to inherently complex processes that combine biological systems with electrochemical and physical ones. This complexity only increases when we are trying to then understand the role of EEU capable microbes in their natural environment or to build more efficient bioelectrochemical reactors for microbial electrosynthesis.
Both approaches require solutions from a variety of disciplines spanning microbiology, genetics, geochemistry, electrochemistry, materials science, and engineering. New tools and methods that can combine and measure the different aspects of BESs are required for us to get closer to a more complete understanding of these complicated systems.
Scaling the laboratory to the microbe level
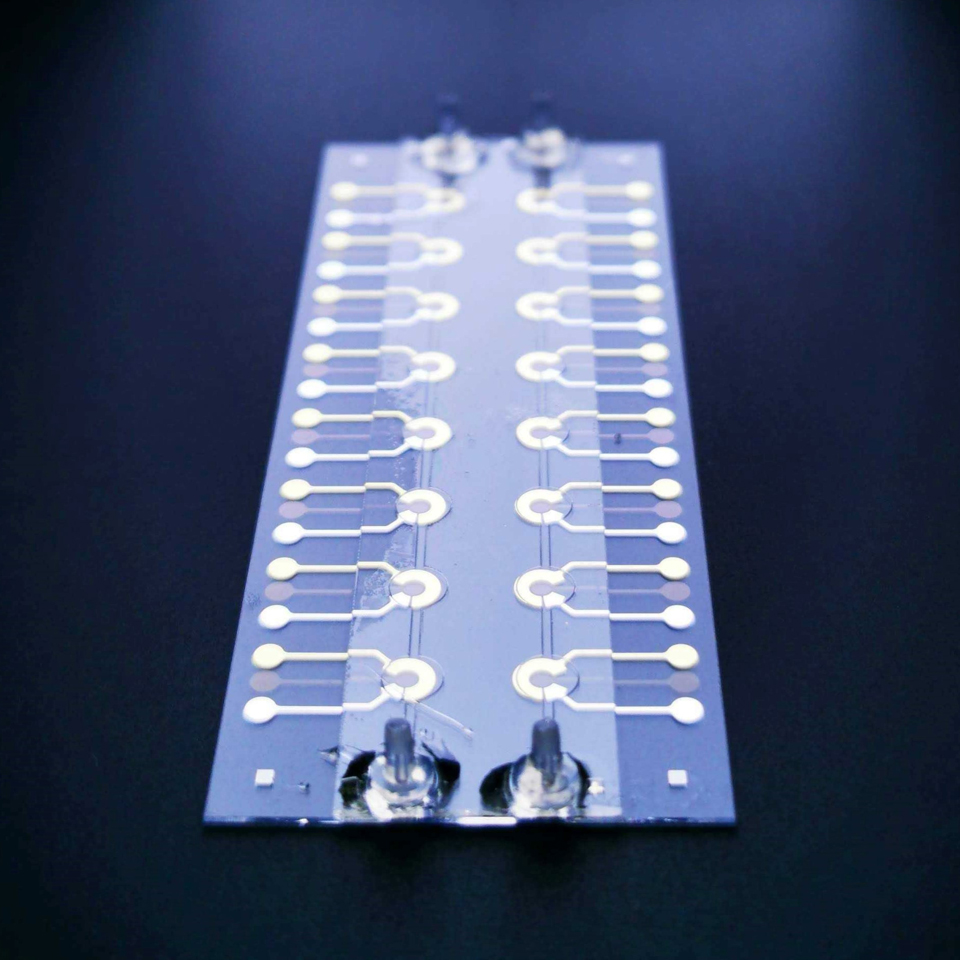
One approach to creating these necessary tools is to get down to the microbial level. By miniaturizing our measurement tools we could control, measure, and observe EEU at scales closer to the microbe size. Scaling down measurement tools to better understand natural and biological phenomena is not a novel approach. Lab-on-a-chip devices that miniaturize and combine several laboratory procedures on a platform that can be held in one’s hand have already accelerated our understanding of human health and other physical and chemical phenomena. These devices not only provide a means for combining different measurement systems but can also bring multiple benefits such as smaller sample volumes, in-situ analysis, shorter processing times, and/or better measurement resolution. While not as prevalent as in the biomedical field, miniaturized microfluidic bioelectrochemical reactors have already been developed in microbiology to study cyanobacteria and microalgae and electroactive bacterial biofilms.
The Bose research group in collaboration with the Meacham lab group develops high-throughput microfluidic bioelectrochemical devices for the study of microbial EEU. By miniaturizing the electrochemical reactor and using transparent electrode materials we are able to combine optical imaging with electrochemical measurements. The microfluidic aspect of our devices allows us to isolate the response of the microbial biofilm from that of planktonic cells and to streamline a chemical probe approach for understanding how different chemical factors affect or inhibit EEU. Moreover, the ability to combine in-situ imaging methods with electrochemical measurements could allow us to have a better understanding of biofilm formation by imaging the microbial biofilms in real time rather than at the end of experiments.
The ability to perform a large number of experiments in parallel on the same chip could also allow for faster screening of better electrode materials. Understanding EEU at the single bacterial cell level may also be possible by miniaturizing the electrode size.
While miniaturization and microfluidics could provide endless avenues for characterizing EEU, their success depends on the collaboration of researchers from diverse STEM fields. Understanding the crucial role electroactive microbes play for our environment is an intrinsically interdisciplinary problem. We can best identify the important questions and design the right tools for finding their answers though a joint multidisciplinary effort.
We would like to thank Karthikeyan Rengasamy for the critical reading of this article.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.