Therapeutic strategies for multiple sclerosis reduce the number of relapses and improve quality of life early in the disease course, nevertheless, neurodegeneration ultimately gives rise to permanent disability
Current treatment strategies for multiple sclerosis (MS) are immunomodulatory by preventing activation, proliferation, or extravasation of immune cells into the central nervous system (i.e. brain, spinal cord, and optic nerve). These therapeutic strategies reduce the number of relapses and improve the quality of life early in the disease course, nevertheless, neurodegeneration ultimately gives rise to permanent disability (1).
A relapse is a clinical event involving the onset of neurological symptoms (clinical disability, green bars, Fig. 1). However, the number of inflammatory events in the CNS, detected by magnetic resonance imaging (MRI activity, arrows, Fig. 1) is far greater than the number of relapses in MS patients. In fact, MRI activity may occur during the preclinical phase before the first sign of clinical disability when most patients are diagnosed with relapsing-remitting MS (Fig. 1). This may explain why immunomodulatory drugs initially reduce relapses and improve quality of life, but long-term disease progression (secondary progressive phase, Fig. 1) proceeds without remedy.
During the secondary progressive phase, clinical disability worsens coincident with decreasing brain volume (red dotted line, Fig. 1) and increasing disease burden (brown line, Fig. 1). To date, there are no therapeutic strategies for the secondary progressive phase of MS, making detection of neurodegeneration essential for the development of effective therapeutic interventions.
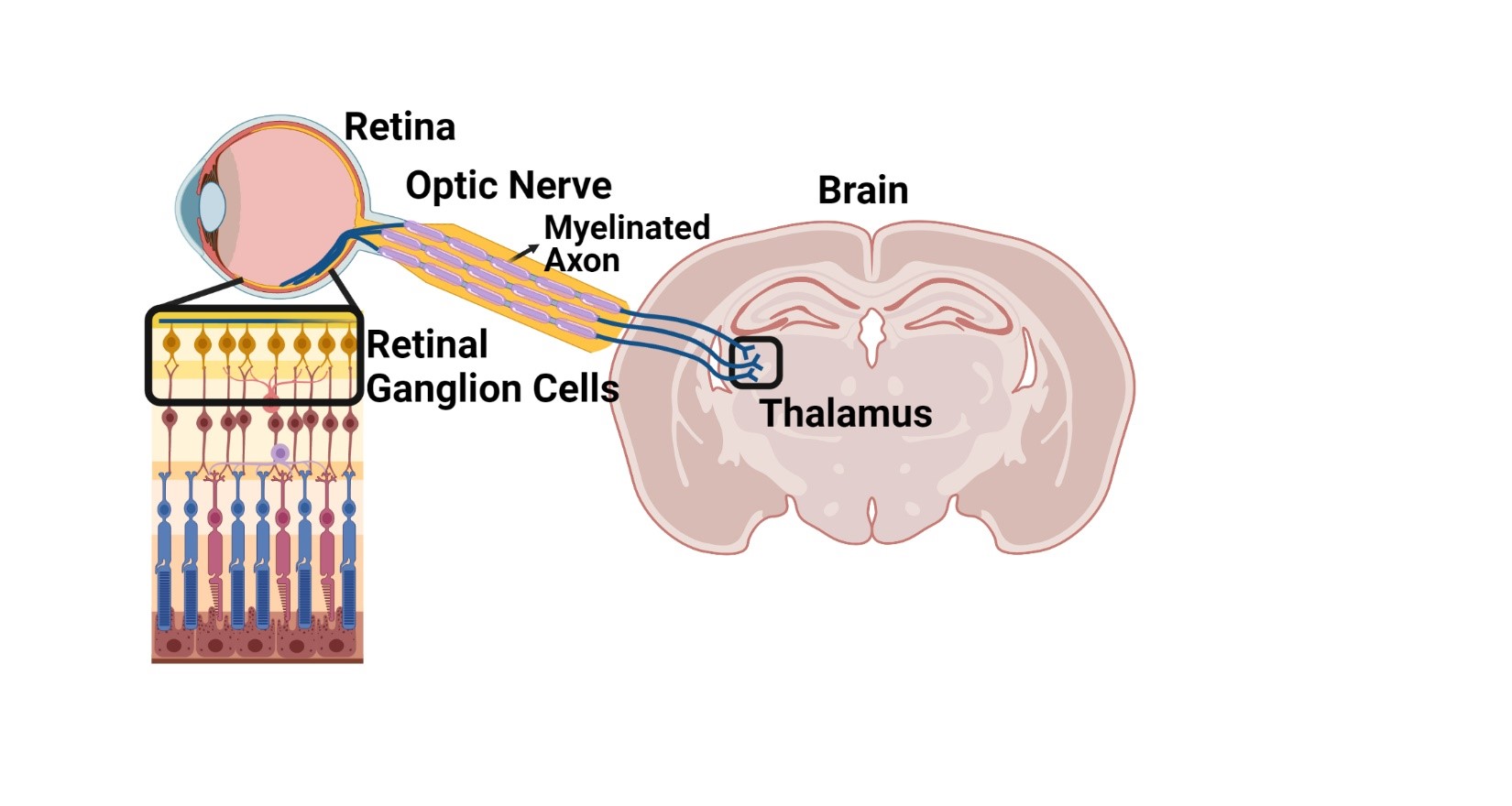
Although MS is initially diagnosed by the detection of white matter lesions by MRI, atrophy in a particular gray matter region of the brain termed the thalamus (Fig. 2) is one of the strongest predictors of worsening disability (2). The thalamus receives input from myelinated axons that arise from white matter structures in the central nervous system, including the optic nerve (Fig. 2), which is important for information processing. Thus, understanding damage to white matter pathways that project to the thalamus will provide important clues to the timing of neurodegenerative processes in MS with important ramifications for neuroprotective strategies.
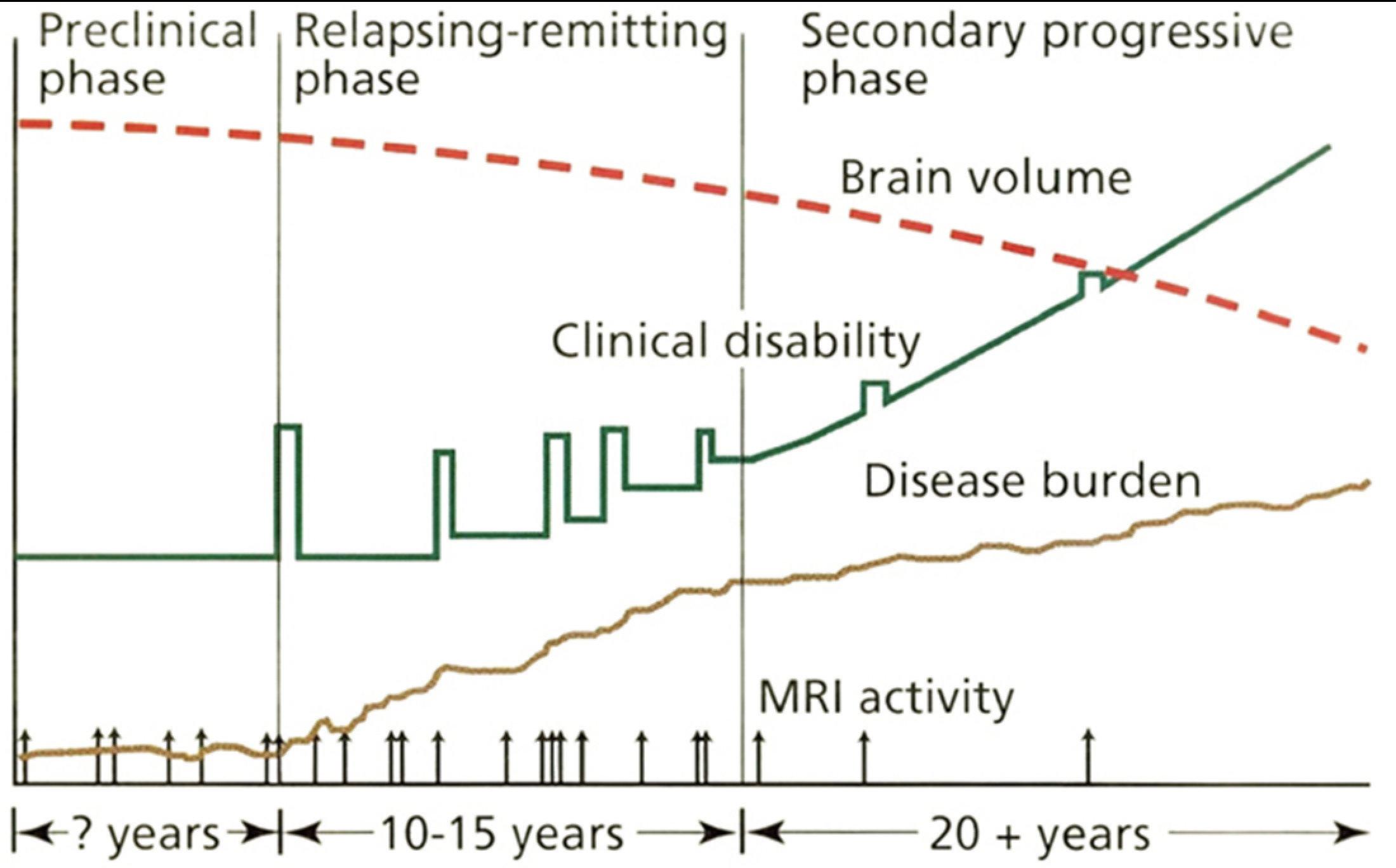
Reprinted with permission from: Fox RJ, Cohen JA. Multiple sclerosis: The importance of early recognition and treatment. Cleve Clin J Med 2001; 68(2):157–171. Copyright © 2001 Cleveland Clinic Foundation. All rights reserved.
How can the retina be used as a biomarker for neurodegeneration in multiple sclerosis?
To address this question the DeSilva Laboratory utilized an in vivo model that is commonly used to test therapeutic strategies for the treatment of MS. The DeSilva Laboratory’s recently published study (3) demonstrates that immune cells infiltrate both spinal cord white matter and the optic nerve (Fig. 2) concomitant with loss of myelinated axons during the acute phase of the disease. Loss of spinal cord neurons or retinal ganglion cells (Fig. 2) retrograde to their respective axons was not observed until the chronic phase of the disease.
These data suggest that neurodegeneration in the visual pathway reflects neurodegeneration occurring in other parts of the CNS. Retinal ganglion cell loss can be monitored using an easily accessible and non-invasive imaging technology called optical coherence tomography (OCT). Using OCT imaging, the DeSilva Laboratory’s published study (3) shows that thinning of the retinal ganglion cell layer was consistent with the loss of retinal ganglion cells observed by histopathological analysis suggesting a sensitive approach to tracking neurodegeneration in MS. Therefore, to explore the relevance of this technology in patients with MS, the DeSilva Laboratory’s study (3) used OCT imaging of retinal ganglion cells compared to MRI measures of atrophy in specific subregions of the thalamus that receive innervation from the optic nerve and spinal cord white matter.
What was discovered when studying patients with relapsing-remitting MS?
Two main findings were revealed in this study of patients with relapsing-remitting MS. First, MRI imaging in both the visual and spinal cord subregions of the thalamus significantly correlated with clinical disability. Second, thinning of the retinal ganglion cell layer by OCT imaging significantly correlated with MRI measures of the aforementioned subregions of the thalamus as well as neuroperformance measures. Of note, these findings were documented in patients without a history of optic neuritis (i.e. visual impairments) indicating that retinal imaging is an important sensitive predictor of neurodegeneration in MS.
Neurodegeneration likely precedes clinical disability and the distinct cellular architecture of the retina provides a sensitive and inexpensive maging technology to monitor neurodegeneration. Longitudinal OCT monitoring may therefore have an important use in addition to MRI and neuroperformance measures throughout the disease course. Elucidating the chronological timeline of neurodegeneration is necessary to develop therapeutic interventions targeting the respective underlying mechanisms early in the MS disease course. OCT monitoring in concert with MRI imaging may also provide a more effective methodology to test the efficacy of future neuroprotective strategies.
Reference
- Mey, G.M., K.R. Mahajan, and T.M. DeSilva, Neurodegeneration in multiple sclerosis. WIREs Mech Dis, 2022: p. e1583.
- Mahajan, K.R., et al., Intrinsic and Extrinsic Mechanisms of Thalamic Pathology in Multiple Sclerosis. Ann Neurol, 2020. 88(1): p. 81-92.
- Mey, G.M., et al., Visual imaging as a predictor of neurodegeneration in experimental autoimmune demyelination and multiple sclerosis. Acta Neuropathol Commun, 2022. 10(1): p. 87.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.